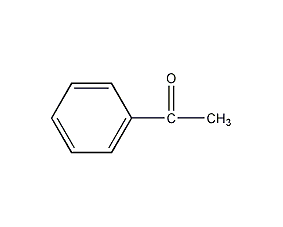
Structural formula
| Business number | 02EP |
|---|---|
| Molecular formula | C8H8O |
| Molecular weight | 120.15 |
| label |
Methyl phenyl ketone, Acetophenone, phenyl methyl ketone, benzoylmethyl, 1-Phenyl-ethanone, Methyl phenyl ketone, Benzoylmethane, Hypnone, Acetyl-benzene, Phenyl methyl ketone, Phenylethanone, plasticizer, spice ingredients |
Numbering system
CAS number:98-86-2
MDL number:MFCD00008724
EINECS number:202-708-7
RTECS number:AM5250000
BRN number:605842
PubChem number:24844956
Physical property data
1. Properties: Colorless or light yellow liquid at normal temperature, flaky crystals at low temperature, with a hawthorn-like aroma.
2. Boiling point (ºC, 101.3kPa): 202.0
3. Melting point (ºC): 20.5
4. Relative density (g/mL, 20 /4ºC): 1.0289
5. Relative density (g/mL, 25/4ºC): 1.0240
6. Relative vapor density (g/mL, air=1): 4.14
7. Refractive index (15ºC): 1.53631
8. Refractive index (n20ºC): 1.5350
9. Viscosity (mPa·s,15ºC): 2.015
10. Viscosity (mPa·s, 25.5ºC): 1.642
11. Viscosity (mPa·s, 30ºC): 1.511
12. Flash Point (ºC, closed): 82
13. Ignition point (ºC): 571
14. Heat of vaporization (KJ/mol, 25ºC): 53.42
15. Heat of evaporation (KJ/mol, b.p.): 38.83
16. Heat of formation (KJ/mol, 25ºC, liquid): 142.60
17. Heat of combustion (KJ/mol , 25ºC, liquid): 4157.1
18. Specific heat capacity (KJ/(kg·K), 30ºC, constant pressure): 1.90
19. Critical temperature (ºC): 456
20. Conductivity (S/m, 16.5ºC): 21×10-8
21. Solubility (%, water): 0.55 p>
22. Vapor pressure (kPa, 25ºC): 0.049
23. Solubility: Slightly soluble in water, miscible with various organic solvents such as alcohol and ether. The dissolving ability is similar to that of cyclohexanone, and can dissolve nitrocellulose, cellulose acetate, vinyl resin, and coumarone.Acetophenone.
2.The vapor of this product has an anesthetic effect and can cause dermatitis. The oral LD50 in rats is 3000mg/kg.
3. It turns orange when dissolved in concentrated sulfuric acid.
4. It is irritating to the eyes. When using it in large quantities, you should wear appropriate protective clothing. If it comes into contact with your eyes, rinse immediately with plenty of water and consult a doctor.
5. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
6. Naturally found in cistus extract, castoreum extract, iris oil, green tea oil, as well as broadleaf tar and bitter poplar oil. .
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and acids, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Galvanized iron drum, net weight: 200kg±1kg.
3. The product should be placed in a cool, dry place during transportation and storage, and should not be close to fire or heat sources.
Synthesis method
1. Under the catalysis of aluminum trichloride, acetophenone can be produced by reacting benzene with acetyl chloride, acetic anhydride or acetic acid. In addition, when ethylbenzene is catalytically oxidized to styrene, acetophenone is produced as a by-product. The main impurities of industrial grade acetophenone include α-methylbenzyl alcohol, phenol, acidic substances, water, etc. It is refined by drying with calcium chloride or sulfuric acid and then fractionating under reduced pressure. Or it can be refined by step-by-step crystallization from the molten state while avoiding light and moisture, or it can also be crystallized and refined with pentane at low temperature. Raw material consumption quota: benzoic acid 1130kg/t, acetic acid 555kg/t.
2. Obtained from the co-distillation of calcium benzoate and calcium acetate.
It is obtained by reacting benzene and acetyl chloride in the presence of aluminum chloride.
Originated from the oxidation of ethylbenzene.
Refining method: The main impurities include α-methylbenzyl alcohol, phenol, acidic substances and water. The general refining method is to dry with calcium chloride or calcium sulfate and then fractionate under reduced pressure. Or crystallize and refine it step by step from its molten state while protecting it from light and moisture. Isopentane can also be used for crystallization and purification at low temperatures. Acetophenone mixed with other liquids is generally recovered by steam distillation and then fractionation.
3.While stirring, slowly add the redistilled acetic anhydride to the refined anhydrous benzene (excess) and anhydrous Reaction in aluminum trichloride:
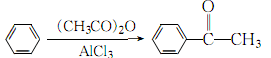
When the generated hydrogen chloride gas no longer escapes, heat in a water bath for 30 minutes to complete the reaction. After cooling slightly, pour into a mixture of concentrated hydrochloric acid and crushed ice to completely dissolve the aluminum salt, then add diethyl ether, let stand and separate the layers, extract the aqueous layer with diethyl ether, combine the extract layer with the organic layer, and use 10% hydrogen Wash with sodium oxide until the washing liquid becomes alkaline, then wash with water, let stand and separate into layers. The organic layer is dried and dehydrated with anhydrous calcium chloride, and then distilled. First, benzene and ether are evaporated, and then distilled under reduced pressure, and collected at 2133 Pa. The 88-89°C fraction is the finished product.
4. Tobacco: BU, 56; BU, 14; OR, 26; FC, 9; FC, BU, OR, 18; FC, 40.
Purpose
1. Used in the manufacture of soaps and cigarettes, and also used as intermediates in organic chemical synthesis, solvents for fiber resins, etc., and plasticizers for plastics. Used as a solvent for cellulose ethers, cellulose esters, resins, preservatives, rubber, medicines, dyes, etc.
2. Used as plasticizer, spice ingredient and pharmaceutical raw material. When used as a solvent, it has the characteristics of high boiling point, stability, and pleasant smell. It is often mixed with ethanol, ketones, esters and other solvents.
3.Used in organic synthesis such as spices. Used as solvent and extraction agent. Also used as a reference material for chromatographic analysis.

 微信扫一扫打赏
微信扫一扫打赏

