background and overview[1]
2,6-dichloro-3-(trifluoromethyl)benzoic acid is an organic intermediate that can be prepared from 2,4-dichloro-1-(trifluoromethyl)benzoic acid through a two-step reaction. . starting from 2,4-dichlorotrifluorotoluene, the reaction was continuously treated with butyllithium and dry ice to obtain 2,6-dichloro-3-(trifluoromethyl)benzoic acid with a yield of 75%.
preparation method[1]
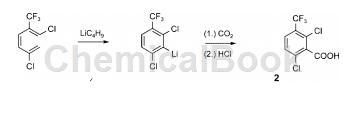
a solution of 2,4-dichloro-1-(trifluoromethyl)benzene (3.6 ml, 5.4 g, 25 mmol) and butyllithium (25 mmol) in tetrahydrofuran (25 ml) and hexane (15 ml) was heated at -75 °c for 45 minutes. pour the dark purple mixture onto an excess of freshly crushed dry ice wrapped in tetrahydrofuran (25 ml). after evaporation of the solvent, the residue was partitioned between water (10 ml) and hexane (10 ml). acidify the aqueous layer with concentrated hydrochloric acid (10 ml) and extract with diethyl ether (3 × 25 ml). crystallization from hexane gave colorless needles; melting point 95-97°c; yield: 4.87 g (75%). 1 h nmr: δ = 7.72 (d, j = 8.6hz, 1h), 7.50 (d, j = 8.6hz, 1h) ppm. 13c nmr: δ= 169.5 (s), 135.4 (s), 135.0 (s), 130.4 (q, j = 2 hz), 129.1 (q, j = 5 hz), 128.1 ( s), 127.9 (q, j = 32hz), 122.1 (q, j = 274hz) ppm. c8h3cl2f3o2 (259.01): calculated c 37.10, h 1.17; found c 37.17, h 1.18.
main reference materials
[1] masson e , marzi e , cottet f , et al. metalation and derivatization of all six dichlorobenzotrifluorides:site selectivities[j]. european journal of organic chemistry, 2005, 2005(20):8.

 微信扫一扫打赏
微信扫一扫打赏

