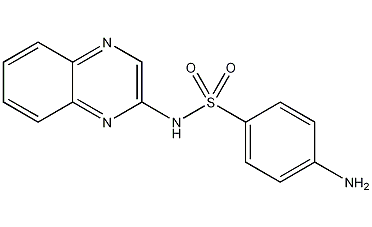
Structural formula
| Business number | 01AC |
|---|---|
| Molecular formula | C14H12N4O2S |
| Molecular weight | 300.34 |
| label |
N-2-quinoxalinyl-4-aminobenzenesulfonamide, 4-Amino-N-(2-quinoxalinyl)benzenesulfonamide, antibacterial growth promoter |
Numbering system
CAS number:59-40-5
MDL number:MFCD00055406
EINECS number:200-423-2
RTECS number:WP2100000
BRN number:290026
PubChem number:24869211
Physical property data
1. Properties: light yellow crystal or powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Not determined
5. Boiling point (ºC, normal pressure): Not determined
6. Boiling point (ºC, 5.2kPa): Not determined Determined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water. The pH value of its 1% solution is approximately 10.
Toxicological data
1. Acute toxicity: Rat oral LD50: 1370mg/kg; Mouse oral LD50: 15mg/kg 2. Other multiple dose toxicity: Rat oral TDLo: 9100mg/kg/26W-I; Dog oral TDLo: 14000mg /kg/14D-I
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 106
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 442
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalency�Number of units: 1
Properties and stability
1. Molar refractive index: 80.70
2. Molar volume (m3/mol): 201.3
3. Isotonic specific volume (90.2K): 609.3
4. Surface tension (dyne/cm): 83.7
5. Polarizability (10-24cm3): 31.99
Storage method
This product should be kept sealed.
Synthesis method
Add 600 g (3.64 mol) of 1.2-chloroquinoline, 688 g (4.00 mol) of sulfonamide, and 800 g K2CO3 into a 2500 mL four-neck flask, heat, react for 3 hours, add water and boil for 5 min, cool, filter, and obtain the filtrate Carefully acidify with dilute hydrochloric acid to pH=5.5, cool and filter, and dry the filtrate to obtain 1050 g of light yellow powdery solid, which is the product. The yield is 98% and the purity is 98.5%.
2.Using o-phenylenediamine as raw material, it first reacts with sodium cyanide and formaldehyde in an acidic medium, and then undergoes cyclization and oxidative dehydrogenation. , and finally condensed and hydrolyzed to obtain the product. Products can also be made from the reaction of o-phenylenediamine and chloroacetic acid, followed by oxidation and condensation.
Purpose
1. Veterinary coccidiostatic agent. Sulfaquinoxaline has the strongest effect on Eimeria giantis, Brucella brucei, and Eimeria aeruginosa in chickens, but it is less effective against tender and poisonous Eimeria, and usually requires a higher concentration to be effective. Therefore, this product is usually used in combination with amprolium or antibacterial synergists to expand the anti-insect spectrum and enhance the anti-coccidial effect. 2.Broad-spectrum antibacterial agent for animals. Sulfaquinoline has the strongest effect on Eimeria giganteum, Eimeria brucelli and Eimeria aeruginosa in chickens, but is less effective against tender and poisonous Eimeria, and usually requires a higher concentration to be effective. Sulfaquinoline has a narrow anti-insecticide spectrum and high toxicity, so it should be used in combination with other anti-coccidial drugs (such as amprolium or antibacterial synergists).

 微信扫一扫打赏
微信扫一扫打赏

