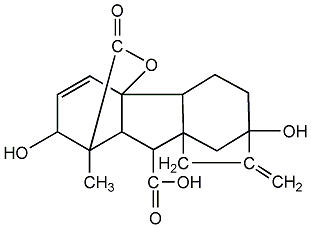
Structural formula
| Business number | 01LL |
|---|---|
| Molecular formula | C19H22O6 |
| Molecular weight | 346 |
| label |
Gibberellin A, gibberellin, nine-twenty, Qibao;, Berelex, Gibreskol, Gibberellin, Gibberellins; GA, Plant Growth Regulator |
Numbering system
CAS number:77-06-5
MDL number:MFCD00079329
EINECS number:201-001-0
RTECS number:LY8990000
BRN number:54346
PubChem number:24895317
Physical property data
1. Appearance: White crystalline powder
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, Air=1): Uncertain
4. Melting point (ºC): 233~235℃
5 . Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (% ,V/V): Uncertain
19. Solubility: Easily soluble in alcohol, acetone, ethyl acetate, sodium bicarbonate solution and phosphate buffer with pH 6.2, insoluble in water and Ether
20. Stability: unstable, easy to decompose when exposed to alkali, turns dark red when exposed to sulfuric acid
Toxicological data
It has estrogen-like activity. After intraperitoneal injection of 35 mg/(kg·d) for 7 days, it can correct uterine atrophy in ovariectomized mature female rats. This product is also a plant hormone [5].
Ecological data
None yet
Molecular structure data
5. Molecular properties��Data:
1. Molar refractive index: 85.78
2. Molar volume (cm3/mol): 231.9
3. Isotonic specific volume (90.2K): 675.4
4. Surface tension (dyne/cm): 71.8
5. Polarizability (10-24cm3): 34.00
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.2
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 104
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 772
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 8
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. No teratogenic or mutagenic effects.
2.Aqueous solution is acidic, relatively stable in acidic and weakly acidic solutions, and easily decomposes when exposed to alkali. It has an aromatic smell after dissolving. Gibberellin can be stored for a long time in a dry state.
Storage method
1. Powders are packed in plastic bags, and liquids are packed in brown glass bottles. Store at low temperature and dry place.
Synthesis method
1. It is obtained by fermenting the culture solution of gibberella fujikuroi into sterile air and then refining it. The inoculum amount is 4% of the final fermentation tank liquid, and the temperature is 25 to 30°C, with constant stirring and ventilation. During the stirring process, octadecyl alcohol is used as an anti-foaming agent. After fermentation, the solution was precipitated with barium hydroxide and filtered. Then on the cation exchange resin, it is adsorbed at pH=50 and eluted with NH3 or other alkaline buffer, and then refined.
2.Gibberellins can be produced by fermentation. Gibberella is used to ferment in a culture medium such as bran, sucrose and inorganic salts. Gibberellus metabolizes gibberellin, and the fermentation liquid is extracted and concentrated with a solvent.
3.Nowadays, it is produced by fermentation method. The fermentation broth is passed through an ion exchange resin Amberlite XAD-4 column, washed with water until clean, and then eluted with a 90% acetone aqueous solution. The eluate is collected and concentrated and dried. The gibberellic acid content in the finished product is more than 90%.
Purpose
1. This product has a significant yield-increasing effect on cotton, grapes and vegetables. Can promote seed germination, plant growth and early flowering. It can be used by smearing, seed dressing, root dipping, spraying, etc. When preparing gibberellin powder, you can first dissolve it in a small amount of alcohol or white wine.
2.Highly effective plant growth regulator. It can promote crop growth and development, advance maturity, improve quality and increase yield. It can quickly break the dormancy of organs such as seeds, tubers and bulbs, promote the shedding of buds, flowers, bolls and fruits, improve the fruit setting rate or form seedless fruits. It can be used on rice, wheat, cotton, fruit trees, vegetables and other crops to promote their growth, germination, flowering and fruiting. Whether sprayed, applied or dipped in roots, gibberellin can increase the yield of different crops. However, if too much gibberellin is applied, the plants will have yellow and slender branches, which are chlorosis and leggy, which will in turn affect the yield. Gibberellins are also used to make malt from barley. It also promotes the developmentof insects.
3.Gibberellic acid has estrogen-like activity, which can promote the extension of cell life and stimulate cell division. When used in hair products, it can promote scalp blood circulation and reduce hair loss. The generation of dandruff and stimulation of hair growth preventhair loss. Used in skin care products, it can inhibit the production of melanin, lighten the color of moles and freckles on the skin, and whiten the skin. Gibberellic acid is safe for use in cosmetics.

 微信扫一扫打赏
微信扫一扫打赏

