Background and overview[1]
6-Chlorobenzoxazole can be used as a pharmaceutical synthesis intermediate. If 6-chlorobenzoxazole is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel unwell; if there is eye contact , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1]
6-Chlorobenzoxazole can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
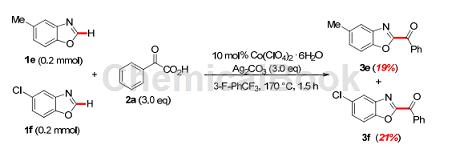
The specific steps are as follows: Add 6-chlorobenzoxazole (0.2mmol), α-oxocarboxylic acid 2 (0.6mmol), Co(ClO4)2·6H2O (7.3mg) to a 35mL oven-dried pressure tube. , 0.02mmol), Ag2CO3 (165.4mg, 0.6mmol). ) and 3-F-PhCF3 (2.0mL). The tube was then sealed and stirred vigorously at 170°C for 24 hours. After cooling to room temperature, the reaction mixture was diluted with DCM (20 mL), filtered through a pad of Celite, and the filtrate was concentrated in vacuo. The residue was purified by silica gel flash chromatography (1% ethyl acetate in petroleum ether, v/v) to afford the desired products (3e) and (3f).
(5-Methylbenzo[d]oxazol-2-yl)(phenyl)methanone (3e): white solid, 25.0 mg, yield: 53%. 1HNMR (400MHz, CDCl3) δ8. 56(d, J=8.0Hz, 2H), 7.75–7.70(m, 2H), 7.60(t, J=7.6Hz, 3H), 7.39(d, J=8.4Hz, 1H), 2.55(s, 3H ).13CNMR (100MHz, CDCl3) δ180.7, 157.3, 148.7, 141.0, 135.8, 135.1, 134.2, 131.0, 129.9, 128.6, 121.9, 111.2, 21.6.
(5-Chlorobenzo[d]oxazol-2-yl)(phenyl)methane (3f): white solid, 44.8mg, yield: 87%.1HNMR (400MHz, CDCl3) δ8.57– 8.55(m, 2H), 7.96(d, J=2.0Hz, 1H), 7.73(t, J=7.6Hz, 1H), 7.68(d, J=8.8Hz, 1H), 7.61(t, J=7.6 Hz, 2H), 7.55 (dd, J=8.8, 2.0Hz, 1H).13CNMR (100MHz, CDCl3) δ180.2, 158.1, 149.0, 141.7, 134.7, 134.6, 131.3, 131.0, 128.9, 128.7, 122.1, 112.7 .
Main reference materials
[1] Cobalt-Catalyzed Decarboxylative 2-Benzoylation of Oxazoles and Thiazoles with alpha-Oxocarboxylic Acids

 微信扫一扫打赏
微信扫一扫打赏

