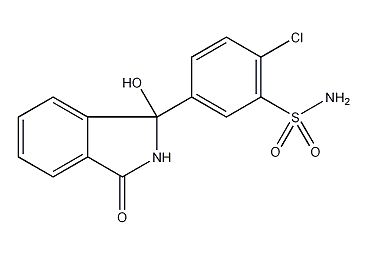
Structural formula
| Business number | 01LQ |
|---|---|
| Molecular formula | C14H11ClN2O4S |
| Molecular weight | 338.77 |
| label |
2-Chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1H-isoindol-1-yl)benzenesulfonamide, 1-Oxo-3-(4′-chloro-3′-sulphamoylphenyl)-3-hydroxyisoindoline, 1-Keto-3-(3′-sulfamyl-4′-chlorophenyl)-3-hydroxyisoindoline, 3-Hydroxy-3-(4-chloro-3-sulfamylphenyl)phthalimidine, Chlorphthalidolone |
Numbering system
CAS number:77-36-1
MDL number:MFCD00036257
EINECS number:201-022-5
RTECS number:DB1556000
BRN number:None
PubChem number:24892527
Physical property data
1. Appearance: white crystalline powder.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density ( g/mL,Air =1): No OK
4. Melting point (ºC):: 224-226℃
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Molar volume (m3/mol ): 211.5
3. isotonic specific volume (90.2K):617.7
4. Surface Tension (dyne/cm):72.7
5. Polarizability(10-24cm3): 32.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 118
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 564
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
this The product should be sealed and stored in a cool place.
Synthesis method
By Phthalic anhydride and chlorobenzene are added, nitrated and reduced to obtain phthalic anhydride (3-Amino– 4-Chlorobenzoyl) benzoic acid, then diazotization, displacement, chlorosulfonation, and finally cyclization and amination.
Purpose
Applicable For cardiac, renal, hepatic and other edema diseases, combined use with antihypertensive drugs can increase the efficacy. The preparation is a tablet.
amily: ‘Times New Roman’; mso-hansi-font-family: ‘Times New Roman'”>amino-4-chlorobenzoyl)benzoic acid, then diazotization, displacement, chlorosulfonation, and finally cyclization and amination.
Purpose
Applicable For cardiac, renal, hepatic and other edema diseases, combined use with antihypertensive drugs can increase the efficacy. The preparation is a tablet.

 微信扫一扫打赏
微信扫一扫打赏

