Background and overview[1]
O-chlorobenzaldehyde oxime is an organic oxime compound and can be used as a pharmaceutical synthesis intermediate. If o-chlorobenzaldehyde oxime is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes come into contact, seek medical attention. Separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The synthesis of o-chlorobenzaldehyde oxime is as follows:
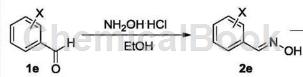
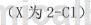
The specific steps are as follows: Weigh 28.10g (0.20mol) of raw material 1e (o-chlorobenzaldehyde) into a 500mL single-neck flask, add 200mL of absolute ethanol to it, and then add 20.85g (0.30mol) of hydroxylamine hydrochloride Dissolve in 70 mL of water, neutralize with sodium carbonate and slowly add it dropwise into the reaction bottle. After the dropwise addition is completed, reflux and stir for 2 hours. TLC will follow the reaction progress. After the raw materials have completely reacted, pour the reaction solution into water. A large amount of white solid was precipitated. After filtering, washing with water and drying, 29.73g of product 2e was obtained with a yield of 95.6%. The m.p. of the product o-chlorobenzaldehyde oxime is 75~77℃, and the nuclear magnetic data is: 1HNMR (600MHz, CDCl3/TMS) δ: 7.35~7.43 (m, 2H , Ar-H), 7.50 (d, 1H, J=8.0Hz, Ar-H), 7.81 (d, 1H, J=8.0Hz, Ar-H), 8.36 (s, 1H, CH=N), 11.63 (s, 1H, OH).
Apply[1]
O-chlorobenzaldehyde oxime can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
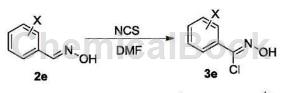
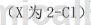
The specific steps are as follows: Add 15.55g (0.10mol) raw material 2e (o-chlorobenzaldehyde oxime) to a 250mL single-neck flask, add 150mLDMF to it, stir to dissolve, and then add 16.02g ( 0.12 mol) N-chlorosuccinimide, dried and protected, reacted at 50-60°C for 3 hours, followed the reaction progress by TLC, and after the raw materials reacted completely, lower the reaction solution to room temperature and seal it for storage.
Main reference materials
[1]CN201610973367.0 A class of substituted aryl bisoxazole arylamidine compounds, their preparation methods and applications

 微信扫一扫打赏
微信扫一扫打赏

