background and overview[1]
3-phenyl-4-methoxyaniline hydrochloride is an amine compound that can be used as a pharmaceutical synthesis intermediate. if 3-phenyl-4-methoxyaniline hydrochloride is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. if discomfort occurs , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
preparation method[1]
3-phenyl-4-methoxyaniline hydrochloride can be used as a pharmaceutical synthesis intermediate. if the following reaction occurs:
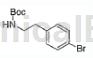
1) add 8.6g (39.4mmol) di-tert-butyl dicarbonate to a solution of 7.03g (35.1mmol) 4-bromophenethylamine (sigma-aldrich) in 60mlthf. after 10 minutes, the solution was concentrated under reduced pressure and the residue was partitioned between saturated aqueous sodium bicarbonate solution and ethyl acetate. the ethyl acetate phase was washed with brine, dried over mgso4, filtered and concentrated to give the intermediate as a white solid.
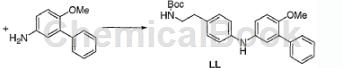
under nitrogen, mix the compound prepared in step 1 (5.0g, 16.7mmol) with toluene (80ml), and add 3-phenyl-4-methoxyaniline hydrochloride (4.3g, 18.3mmol) to a slurry forms. add 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (1.6g, 2.5mmol), then tris(dibenzylideneacetone)dipalladium(0) (760mg, 0.83mmol) ), and finally sodium tert-butoxide (5.3 g, 55 mmol) was added. the mixture was heated at 90°c for 150 minutes and then cooled to room temperature. water (150 ml) was added, followed by ethyl acetate (150 ml), and the phases were separated. the aqueous layer was extracted with ethyl acetate (150 ml), and the combined organics were washed three times with 0.5 m sodium bisulfate (200 ml), once with saturated sodium bicarbonate (150 ml), and twice with saturated sodium chloride (150 ml). the organic matter was dried over magnesium sulfate (50g), and the volatiles were removed in vacuo to obtain n-tert-butoxycarbonyl-2-[4-(3-[phenyl-4-methoxyphenyl]aminophenyl]ethylamine ( ll) (8.4g) was used without further purification.
main reference materials
[1](wo2003042164)arylanilinebeta-2adrenergicreceptoragonists

 微信扫一扫打赏
微信扫一扫打赏

