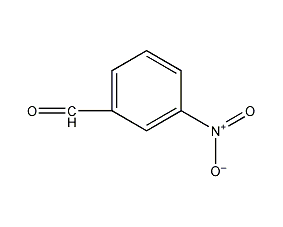
Structural formula
| Business number | 02G5 |
|---|---|
| Molecular formula | C7H5NO3 |
| Molecular weight | 151.12 |
| label |
3-Nitrobenzaldehyde, m-nitrobenzaldehyde, 3-Nitro-benzaldehyd, 3-Nitrobenzaldehyle, 5-Nitrobenzaldehyde, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:99-61-6
MDL number:MFCD00007249
EINECS number:202-772-6
RTECS number:CU7250000
BRN number:386795
PubChem number:24886451
Physical property data
1. Properties: light yellow crystalline powder
2. Relative density (20/4℃): 1.2792
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): 58~59
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 3.07kPa): 164
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation Degree (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20.2ºC): Undetermined
p>
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble In ethanol, ether, chloroform, benzene and acetone. It is almost insoluble in water and can evaporate with water evaporation.
Toxicological data
1. Acute toxicity: Mouse peritoneal cavity LD:> 500mg/kg; mice intravenous injection LD50: 180mg/kg; 2. Cyromicity: mutant microbi test: bacteria-mouse typhoid Salmonella, 600 μg/plane; DNA repair test: DNA repair test: DNA repair test: DNA repair test: Bacillus subtilis, 5mg/disc;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 39.55
2. Molar volume (cm3/mol): 112.9
3. Isotonic specific volume (90.2K ): 307.8
4. Surface tension (dyne/cm): 55.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Pole Transformation rate: 15.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 164
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, and strong alkali.
2.Avoid inhalation, ingestion and skin contact, and wear protective glasses, protective clothing and protective gloves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should also be available to contain spills. Packed in 25kg cardboard drum (lined with double-layer plastic bags), stored in a cool, dry place, sealed container, away from strong oxidants and strong alkali.
Synthesis method
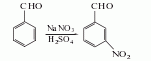
Using benzaldehyde as raw material, nitrification with potassium nitrate, sodium nitrate or nitric acid in the presence of sulfuric acid can produce m-nitrobenzaldehyde, with yields of approximately 80%, 60% and 75% respectively. Add sulfuric acid to the reaction kettle, add sodium nitrate while stirring, heat to 70°C to dissolve it all, cool to 5°C, add benzaldehyde dropwise, complete the addition at 5-10°C, react for 1 hour, and slowly add the reactants to In crushed ice, stir thoroughly to precipitate completely, filter out the precipitate, wash with sodium carbonate at 10°C to remove the acid, filter to dry, wash with alcohol to remove o-nitro compounds, and dry under reduced pressure at low temperature to obtain this product.
Purpose
Used as an intermediate in the organic synthesis of dyes, surfactants, and pharmaceuticals. In the pharmaceutical industry, it is used in the production of calcium iopperate, iopanoic acid, calcium cholephanidine, m-hydroxybitartrate, nifedipine, etc.

 微信扫一扫打赏
微信扫一扫打赏

