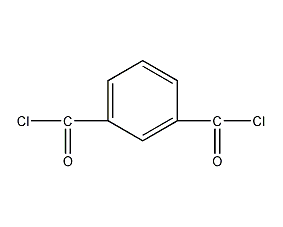
Structural formula
| Business number | 02G7 |
|---|---|
| Molecular formula | C8H4Cl2O2 |
| Molecular weight | 203.02 |
| label |
(iso)phthalyl dichloride, Isophthaloyl dichloride, Isophthaloyl chloride, Isophthalyl dichloride, isophthaloyl chloride, 1,3-Benzenedicarboxylic acid,dichloride, 1,3-Bis(chlorocarbonyl)benzene, Chlorured’acidebenzodicarboxylique-1,3, Chlorured’isophthaloyle, Dichlorid kyseliny isoftalove, Dichloridkyselinyisotalove, Isophthalic acid chloride, Isophthalic chloride |
Numbering system
CAS number:99-63-8
MDL number:MFCD00000678
EINECS number:202-774-7
RTECS number:NT2625000
BRN number:638342
PubChem number:24881369
Physical property data
1. Character: colorless or light yellow crystal
2. Density (g/mL, 20℃): 1.388
3. Relative vapor density (g/mL, Air = 1): 6.9
4. Melting point (ºC): 41
5. Boiling point (ºC, normal pressure): 276
6. Boiling point ( ºC, kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): 180
9. Specific rotation ( º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 25ºC): 0.03
12. Saturated vapor pressure (kPa, 25ºC): 3.9×10-3
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC) : Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Dissolved in Benzene, carbon tetrachloride.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 200 mgREACTION SEVERITY, moderate reaction; Standard Dresser test: rabbit eye contact, 40 mgREACTION SEVERITY, slight reaction; 2. Acute toxicity: Rat oral LD50.�2200mg/kg; Rat inhalation LC: >31mg/m3/4H; Mouse oral LD50: 2221mg/kg; Rabbit oral LD50: 1175mg/kg; Rabbit skin contact LD50: 1410mg/kg; 3. Other multiple doses Toxicity: Rat oral TDLo: 1817mg/kg/22W-I; Rat oral TDLo: 10mg/kg/2W-I;
Ecological data
This substance is harmful to the environment. Do not let this substance enter the environment. Special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 46.74
2. Molar volume (cm3/mol): 142.2
3. Isotonic specific volume (90.2K ): 374.9
4. Surface tension (dyne/cm): 38.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 183
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with moist air. Avoid contact with strong alkalis, oxidants, water, and alcohols.
Storage method
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from alkalis, oxidants, alcohols, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
There are thionyl chloride method, phosphorus pentachloride method, phosgene method, dimethyl isophthalate chlorination method, and isophthalic acid chlorination method. The phosgene method is often used, and the phosgenation reaction is usually carried out in the presence of a formamide catalyst. If isophthalic acid is first converted into an intermolecular acid anhydride, and then the phosgenation reaction is carried out, and isophthaloyl chloride itself is used as the reaction solvent, a higher yield can be obtained.
Purpose
Can be used to synthesize aramid, polyacrylate, high temperature resistant resin, dyes, pigments, medicines and pesticides, etc. Can be used as raw material for aromatic fibers.

 微信扫一扫打赏
微信扫一扫打赏

