Background and overview[1-2]
Phenoxycarboxylic acid herbicides are an important class of herbicides. They are widely used in agriculture due to their advantages of fast weeding speed and wide herbicidal spectrum. The necessary conditions for the structure of phenoxycarboxylic acid active compounds include: a benzene ring, substituted by an oxygen atom on the ring; an aliphatic chain connected to the oxygen atom and a carboxyl group; the benzene ring contains different substituents, among which the 2nd, Compounds substituted at position 4 are the most active. 2-Methyl-4-chlorophenoxyacetic acid is a carboxylic acid derivative. It is used as a herbicide and plant growth agent.
Structure
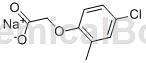
Apply[3]
2-Methyl-4-chlorophenoxyacetic acid is used as a herbicide and plant growth agent in agriculture, and is often processed into sodium salt (2-methyl-4-chlorophenoxyacetic acid), ammonium salt or ester liquid, powder, emulsion, ointment, etc. It is suitable for rice, wheat and other dryland crops to control three-edge grass, duckweed, Alisma, arrowroot and other broad-leaf weeds.
In addition, 2-methyl-4-chlorophenoxyacetic acid is often used in combination with other ingredients, such as preparing a herbicide containing 2-methyl-4-chlorophenoxyacetic acid and cyclohexenone. Composition, the active ingredients of the herbicide composition are: 2-methyl-4-chlorophenoxyacetic acid and cyclohexenone herbicides, said 2-methyl-4-chlorophenoxyacetic acid and said The mass ratio of cyclohexenone herbicides is 1:10~10:1. Preferably, the mass ratio of the 2-methyl-4-chlorophenoxyacetic acid and the cyclohexenone herbicide is 1:6~6:1; most preferably, the 2-methyl-4- The mass ratio of chlorophenoxyacetic acid and the cyclohexenone herbicide is 1:1~6:1.
The dosage form of the herbicide composition of the present invention can be any dosage form permitted by pesticides. The herbicide composition of the present invention shows good synergistic effect within a certain proportion range. The herbicidal effect of the composition is significantly improved compared to a single agent. At the same time, the herbicide dosage and cost are reduced, the residue is reduced, and the pesticide burden is reduced. Potential threats to the environment.
Preparation[1-2]
Method 1: Add 69.80g potassium carbonate with 99% purity and 109.23g toluene to 109.23g o-cresol with a purity of 99%, heat and reflux and dehydrate until the moisture is ≤0.5%, and add 152.14 dropwise to it at 120°C. g n-butyl chloroacetate with a purity of 99% was allowed to react. After the dropwise addition, the reaction was kept at this temperature for 0.5 h, cooled to 30°C, filtered, and an appropriate amount of toluene was added to wash the filter cake, and then dried to obtain potassium chloride and The crude n-butyl o-tolylacetate containing toluene was distilled to recover toluene, and 230.89g of n-butyl o-tolylacetate was obtained with a content of 95.9%. To the n-butyl o-tolylacetate obtained by distillation, add 1.04g of ferric chloride with a purity of 99% and 1.50g of tert-butyl methyl sulfide with a purity of 99%, and add 118.46g of thionyl chloride with a purity of 99% dropwise at 40°C. Let it react. After the addition is completed, the reaction is kept at this temperature for 0.5h to obtain 260.13g of n-butyl 4-chloro-2-methylphenoxyacetate, with a content of 98.01%. To the obtained n-butyl 4-chloro-2-methylphenoxyacetate, add 143.26g of 32% sodium hydroxide and react at 70°C for 4 hours. At the same time, the alcohol generated by the reaction is evaporated. After the reaction is completed, the temperature is cooled to room temperature and filtered. The cake was dried to obtain 223.74g of 4-chloro-2-methylphenoxyacetic acid sodium salt (2-methyl-4-chlorophenoxyacetic acid), with a content of 98.5% and a total yield of 99.00% based on o-cresol.
Method 2: Method for synthesizing high-content 2-methyl-4-chloric acid and sodium salt:
1) Chlorination reaction: Turn on the tail gas absorption system. In the 2000L enamel reactor, vacuum inhale 700 kg of molten o-cresol, and pump 900 kg of sulfuryl chloride into the metering tank for later use. Start stirring and use chilled brine to cool to room temperature (25°C) and start adding sulfuryl chloride dropwise. The addition will be completed in about 5 hours. After the dropwise addition is completed, the reaction liquid is obtained. The temperature of the reaction liquid is raised to about 45°C and the hydrogen chloride and sulfur dioxide are driven out under vacuum for 30 minutes to obtain 925 kilograms of 4-chloro-o-cresol with a content of 98%. The generated tail gas hydrogen chloride is passed through a multi-stage falling film absorption tower to produce hydrochloric acid for subsequent processes. After the sulfur dioxide is dried, it passes with chlorine through a synthesis tower equipped with a catalyst, and then is cooled to obtain sulfuryl chloride for reuse.
2) Condensation reaction: Production of sodium chloroacetate: In a 3000L enamel reaction kettle, put 800 kg of chloroacetic acid, add 1250L of water, stir and dissolve, add anhydrous sodium carbonate in batches below 20°C, and adjust the pH value To about 6.5, approximately 450 kilograms of sodium carbonate are consumed to obtain��Qualified sodium chloroacetate solution, and then transport the qualified sodium chloroacetate solution to the sodium chloroacetate metering tank during the condensation process for later use. Add 860 kilograms of 30% sodium hydroxide solution to the 5000L enamel reaction kettle, start stirring, and put 925 kilograms of qualified chlorinated p-chloro-o-cresol into the reaction kettle, and heat it up to form salt. When the temperature reaches 100°C, start dripping the preparation. A good sodium chloroacetate solution should be added in 1 hour, and the alkaline conditions should be maintained in the kettle with a pH of 10. Reflux and keep warm for 3 hours. The circulating water is cooled to below 35°C, and a large amount of sodium salt is precipitated. Then a separation device is used to separate the material from solid and liquid, and the filter cake is washed twice to obtain 2-methyl-4-chlorophenoxyacetate wet product.
3) Preparation of sodium salt: Directly dry the wet product of 2-methyl-4-chlorophenoxyacetate to obtain 1572 kg of sodium salt with a content of 90.2%.
Main reference materials
[1]CN201810225293.1 Preparation method of phenoxycarboxylate herbicide
[2]CN201610765649.1 A method for synthesizing high-content 2-methyl-4-chloric acid and sodium salt
[3]CN201510884397.X A composition containing 2-methyl-4-chlorophenoxyacetic acid and cyclohexenone herbicides and its use

 微信扫一扫打赏
微信扫一扫打赏

