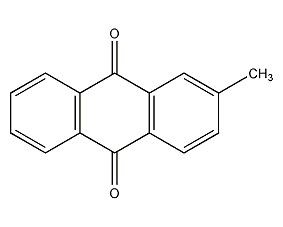
Structural formula
| Business number | 01UQ |
|---|---|
| Molecular formula | C15H10O2 |
| Molecular weight | 222.24 |
| label |
Teak Quinone, β-methylanthraquinone, 2-Methyl-9,10-anthracenedione, β-Methylanthraquinone, Techtoquinone, 2-Methyl-9,10-anthracenedione, Tectochinon, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:84-54-8
MDL number:MFCD00001235
EINECS number:201-539-6
RTECS number:None
BRN number:2050523
PubChem number:24884160
Physical property data
1. Character: yellow crystal
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air =1): Undetermined
4. Melting point (ºC): 175-176
5. Boiling point (ºC, normal pressure): 236~238 (1333pa)
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Undetermined
8. Flash point (ºC): 209
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC) : Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Insoluble in water, soluble in ethanol, ether, benzene and ethyl acetate
Toxicological data
Main irritant effects:
On skin: May cause inflammation.
On eyes: May cause irritation
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 63.49
2. Molar volume (cm3/mol): 175.3
3. Isotonic specific volume (90.2K): 473.1
4. Surface tension (dyne/cm): 53.0
5. Polarizability (10-24cm3): 25.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 347
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product has low toxicity. Can cause allergic eczema, rhinitis, and bronchial asthma. Production equipment should be sealed, the workshop should be well ventilated, and operators should wear protective equipment.
2. Exist in smoke.
Storage method
This product should be sealed and stored in a cool, dry place. Packed in lined plastic bags, outer woven bags or iron drums, and stored in a cool, dry and ventilated place. Keep away from fire and heat sources. Store and transport according to general toxic chemicals regulations.
Synthesis method
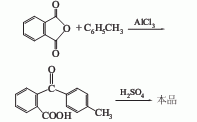
Heat toluene and anhydrous aluminum trichloride, control the temperature at 45-50°C, add phthalic anhydride under external cooling, and keep the mixture warm until the reaction is complete. The reaction mixture was poured into 10% sulfuric acid aqueous solution, and the product-containing slurry was washed with water, separated into layers, and separated. The solvent layer containing the product is mixed with 5% sodium carbonate solution, filtered, washed, and dried to obtain 2-(p-toluoyl)benzoic acid, which is then heated with fuming sulfuric acid at 115°C for 1 hour to close the loop, and the product is poured into cold water. Obtained by filtering, washing and drying.
Purpose
Can be used as dye intermediates, photosensitive agents for photosensitive resins, and raw materials for organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

