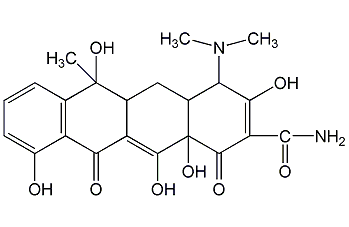
Structural formula
| Business number | 01BH |
|---|---|
| Molecular formula | C22H24N2O8 |
| Molecular weight | 444.43 |
| label |
tetracycline hydrate, 4-(dimethylamino)-1,4,4A,5,5A,6,11,12A-octahydro-3,6,10,12,12A-pentahydroxy-6-methyl-1,11- Dicarbonyl-2-naphthylcarboxamide, tetracycline base, 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide, Fungicide |
Numbering system
CAS number:60-54-8
MDL number:MFCD00151232
EINECS number:200-481-9
RTECS number:QI8750000
BRN number:2230417
PubChem number:24900120
Physical property data
1. Character: Light yellow crystal. Odorless. The color gradually becomes darker when exposed to light. Under vacuum, 60℃Dry8Hours become nothing Water things.
2. Density ( g/mL,25/4℃): Undetermined
3. Relative vapor density ( g/mL,Air =1): Not OK
4. Melting point (ºC): 170~175Decomposition
5. Boiling point (ºC,Normal pressure): Not OK
6. Boiling point (ºC,5.2kPa): Not OK
7. Refractive index: not OK
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation ( º):[α]D25 -257.9°(0.1mol/Lhydrochloric acid)
10. Spontaneous ignition point or ignition Combustion temperature (ºC) : Undetermined
8. Surface charge: 0
9. Complexity: 971
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 5
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 1
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
By Streptomyces aureus Strepto-myces aureofaciensExtracted broad-spectrum antibiotics. Melting point170~ 175℃. Optical rotation-239 (Methanol). Slightly soluble in Water, slightly soluble in ethanol, insoluble in chloroform and acetate ether. Stable in the air, color changes under strong light Deep, rapid failure in alkaline solutions.
Storage method
This product should be sealed in0 Store in a dry place below ℃ and away from light.
Synthesis method
None yet
Purpose
On Gram Positive and negative bacteria, protozoa, mycoplasma, and spirochetes all have antibacterial effects. Oral absorption is incomplete,2~4hPlasma concentration reaches peak value and is widely distributed. Mainly excreted through urine and feces. Currently it is rarely used clinically.
170~175℃. Optical rotation-239 (Methanol). Slightly soluble in Water, slightly soluble in ethanol, insoluble in chloroform and acetate ether. Stable in the air, color changes under strong light Deep, rapid failure in alkaline solutions.
Storage method
This product should be sealed in0 Store in a dry place below ℃ and away from light.
Synthesis method
None yet
Purpose
On Gram Positive and negative bacteria, protozoa, mycoplasma, and spirochetes all have antibacterial effects. Oral absorption is incomplete,2~4hPlasma concentration reaches peak value and is widely distributed. Mainly excreted through urine and feces. Currently it is rarely used clinically.

 微信扫一扫打赏
微信扫一扫打赏

