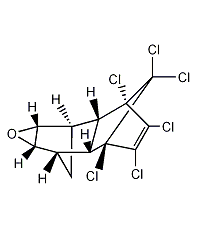
Structural formula
| Business number | 01BK |
|---|---|
| Molecular formula | C12H8Cl6O |
| Molecular weight | 380.91 |
| label |
Oxyconazole, 1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4A,5,6,7,8,8a-octahydro-1,4-endo-5,8 -Exodimethylenenaphthalene, (1aα,2β,2aα,3β,6β,6aα,7β,7aα)-3,4,5,6,9,9-hexachloro-1a,2,2a,3,6,6a,7,7a-eight Hydrogen-[2,3-b]epoxyethylene-2,7:3,6-dimethylenenaphthalene, Dieldrex, Dielmothildrin, Dorytox, Insectlack, Termitox, Organochlorine pesticides |
Numbering system
CAS number:60-57-1
MDL number:MFCD00135591
EINECS number:200-484-5
RTECS number:IO1750000
BRN number:91396
PubChem number:24857518
Physical property data
1. Properties: Industrial product is brown solid. [1]
2. Melting point (℃): 175~177[2]
3. Boiling point (℃) : 330[3]
4. Relative density (water = 1): 1.75[4]
5. Relative Vapor density (air = 1): 13.2[5]
6. Octanol/water partition coefficient: 5.4[6]
7. Solubility: Insoluble in water, methanol, aliphatic hydrocarbons, soluble in acetone, benzene, and carbon tetrachloride. [7]
Toxicological data
1. Acute toxicity[8] LD50: 46mg/kg (rat oral)
2. Irritation No data yet
3. Mutagenicity [9] Microbial mutagenicity: Salmonella typhimurium 1mg/L. DNA inhibition: 400 μmol/L for human HeLa cells. Unprogrammed DNA synthesis: human fibroblasts 1 μmol/L. DNA inhibition: human lymphocytes 100mg/L. Sister chromatid exchange: hamster ovary 40mg/L. DNA damage: mouse fibroblasts 25μmol/L (24h).
4. Teratogenicity [10] The lowest toxic dose (TDLo) of 30,600 μg/kg was administered orally to mice 6 to 14 days after pregnancy, causing Developmental malformations of the central nervous system, eyes, and ears. The lowest toxic dose (TDLo) of 4500 μg/kg was administered orally to mice 6 to 14 days after pregnancy, causing developmental malformations of the musculoskeletal system. The lowest toxic dose (TDLo) of 30mg/kg was administered orally to hamsters on the 8th day after pregnancy, causing developmental abnormalities of the eyes, ears, body wall, and craniofacial face (including nose and tongue).
5. Carcinogenicity [11] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
6. Others[12] The lowest oral toxic dose in rats (TDLo): 336μg/kg (56d , male), affects the prostate, seminal vesicles, Copwer glands, accessory glands, urethra, and mice. The lowest oral toxic dose (TDLo): 30600 μg/kg (gestation 6~14 days), causing abnormal development of the central nervous system, eyes, and ears.
Ecological data
1. Ecotoxicity[13]
LC50: 0.016mg/L (96h) (fathead minnow); 0.008mg/L (96h) (Bluegill sunfish); 0.006mg/L (96h) (Chinchilla salmon); 0.25mg/L (48h) (Flea)
EC50: 1.27mg/ L (24h) (Daphnia)
2. Biodegradability[14]
Aerobic biodegradation (h ): 4200~25920
Anaerobic biodegradation (h): 24~168
3. Non-biodegradability [15] Photooxidation half-life in air (h): 4~40.5
4. Bioconcentration [16] BCF: 4860~14500 (Carp, exposure concentration 0.001mg/L, exposure time 10 weeks); 5390~12500 (carp, exposure concentration 0.001mg/L, exposure time 10 weeks).
Molecular structure data
1. Molar refractive index: 77.48
2. Molar volume (cm3/mol): 205.9
3. Isotonic specific volume (90.2K ): 573.9
4. Surface tension (dyne/cm): 60.2
5. Polarizability (10-24cm3): 30.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 12.5
p>
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 516
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 8
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances[18] Strong oxidizing agent, strong acid
3. Polymerization hazard[19] No polymerization
4. Decomposition products[20] Hydrogen chloride
Storage method
Storage Precautions[21] Store in a cool, well-ventilated special warehouse, and implement the “two people to send and receive, and two people to keep” system. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by oxidizing aldrin with peracetic acid or peroxybenzoic acid.
Purpose
1. Dieldrin is a contact insecticide, non-systemic, has certain specific effects, and has strong contact killing and stomach poisoning activities against most insects.
2. Used as pesticide. [22]

 微信扫一扫打赏
微信扫一扫打赏

