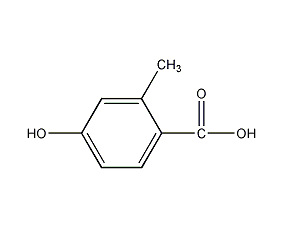
Structural formula
| Business number | 02GF |
|---|---|
| Molecular formula | C8H8O3 |
| Molecular weight | 152.15 |
| label |
Methylparaben, Methyl 4-hydroxybenzoate, 4-Hydroxybenzoic acid methyl ester, Methyl p-hydroxybenzoate, Methylparaben, Preservative additives |
Numbering system
CAS number:99-76-3
MDL number:MFCD00002352
EINECS number:202-785-7
RTECS number:DH2450000
BRN number:509801
PubChem number:24895585
Physical property data
1. Properties: Colorless crystals or white crystalline powder, odorless or slightly pungent odor.
2. Relative density (20℃, 4℃): 1.1208137.2
3. Relative density (25℃, 4℃): 1.1097150.4
4. Melting point (ºC): 131
5. Boiling point (ºC, normal pressure): 275 (decomposition)
6 . Boiling point (ºC, mmHg): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature ( ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, soluble in alcohol, ether, acetone, slightly soluble in benzene and carbon tetrachloride.
20. Freezing point (ºC): 131
Toxicological data
1. Acute toxicity: Rat subcutaneous LD50: >500mg/kg; Mouse oral LC50: >8mg/kg; Mouse peritoneal cavity LD50: 960mg/kg; Mouse subcutaneous LC50: 1200mg/kg; Rabbit oral LD50: 6mg/kg; Guinea pig oral LD50: 3mg/kg; 2. Mutagenicity: Cell generation analysis test: hamster lung, 125mg/L/27H; Cell generation analysis test: hamster fibroblasts, 500mg/L;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 39.88
2. Molar volume (cm3/mol): 116.6
3. Isotonic specific volume (90.2K ): 322.0
4. Surface tension (dyne/cm): 58.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.81
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 4
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 136
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, and strong alkali.
2. Exist in smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage.
2. Equip with corresponding varieties and quantities of fire-fighting equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the esterification of p-hydroxybenzoic acid and methanol. Add excess methanol to dissolve p-hydroxybenzoic acid, and slowly add concentrated sulfuric acid while stirring. Heating to reflux for 10 hours, pour into water to precipitate crystals, and wash with water, sodium carbonate solution, and water to obtain crude product. The finished product is obtained by recrystallizing with water or 25% ethanol. The yield is 85%. Raw material consumption (kg/t) p-hydroxybenzoic acid 1200 methanol 1000
Purpose
Cosmetic preservatives. It is a phenolic preservative and is effective against various molds, yeasts, and bacteria. However, paraben has low bactericidal power. It is usually mixed with ethyl paraben and has good additivity and synergy. Adding amount is 0.1%~1.0%. The antiseptic activity is related to the pH value of the solution. When the pH value is 7, its activity is 2/3 of the original activity; if the pH value is 8.5, the activity is reduced to half of the original activity. It will be bound by some polymer compounds such as methylcellulose, gelatin protein, etc., causing it to lose its preservative activity.

 微信扫一扫打赏
微信扫一扫打赏

