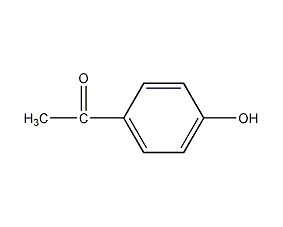
Structural formula
| Business number | 02GT |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
4-Hydroxyacetophenone, p-hexanoylphenol, p-hydroxyacetophenone, 4-Hydroxyacetophenone, 4-Acetylphenol, P-Hydroxyacetophenone, 1-(4-Hydroxyphenyl)-ethanon, 4-Hydroxyacetophenone, 4-Hydroksyacetofenol, 4-Acetylpehnol, p-Acetylphenol, p-hydroxyphenyl ketone |
Numbering system
CAS number:99-93-4
MDL number:MFCD00002359
EINECS number:202-802-8
RTECS number:AM8750000
BRN number:774355
PubChem number:24879002
Physical property data
1. Properties: white powder
2. Density (g/mL, 25℃): 1.109
3. Relative vapor density (g/mL, air=1) : Undetermined
4. Melting point (ºC): 109~111
5. Boiling point (ºC, normal pressure): 147-148 (399.9pa)
6. Boiling point (ºC, 45mmHg): Undetermined
7. Refractive index (n109 D): 1.5577
8. Flash point (ºC): 166
9. Specific rotation (º): Undetermined
10 . Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure ( KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, easily soluble in ethanol and ether.
Toxicological data
1. Acute toxicity: Mouse oral LD50: 1500mg/kg; Mouse peritoneal cavity LD50: 200mg/kg; 2. Reproductive toxicity: Oral TDLo in rats 11 days after conception: 1mg/kgSEX/DURATION;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 38.16
2, Molar volume (cm3/mol): 119.3
3, Isotonic specific volume (90.2K): 307.4
4. Surface tension (dyne/cm): 43.9
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 15.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 7
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 123
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants and water.
2. Exist in tobacco leaves and smoke.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by acylation and translocation of phenol. Mix phenol and acetyl chloride and slowly heat until hydrogen chloride stops escaping to obtain crude phenyl acetate. Add nitrobenzene, add aluminum trichloride under cooling, stir at room temperature for 2-3h after adding. Then pour cold water and add 1:3 hydrochloric acid until clear. Extract with diethyl ether, then use the extraction solution to recover the diethyl ether, and then carry out steam distillation. Nitrobenzene and the by-product o-hydroxyacetophenone are steamed out at any time, and p-hydroxyacetophenone remains in the residual liquid. Extract the residual liquid with diethyl ether, recover the diethyl ether and then cool and crystallize to obtain the crude product, which can be recrystallized with water to obtain the finished product. In the above acylation operation, acetic anhydride can be used instead of acetyl chloride, and phenol and acetic anhydride are heated and refluxed for 3 hours to generate phenyl acetate with a yield of about 83%. Transposition under the action of anhydrous aluminum trichloride can also be done without solvent. In addition, p-hydroxyacetophenone can also be obtained by diazotizing and hydrolyzing p-aminoacetophenone with sodium nitrite.
Purpose
Used in the manufacture of choleretic drugs and other raw materials for organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

