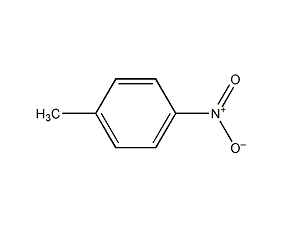
Structural formula
| Business number | 02GY |
|---|---|
| Molecular formula | C7H7NO2 |
| Molecular weight | 137 |
| label |
1-Methyl-4-nitrobenzene, p-methylnitrobenzene, p-Nitrotoluene, 4-Nitrotoluene, 1-Methyl-4-mitrobenaene, 4-Methyl-1-nitrobenzene, Pesticide intermediates; aromatic nitrogen-containing and its derivatives |
Numbering system
CAS number:99-99-0
MDL number:MFCD00007366
EINECS number:202-808-0
RTECS number:XT3325000
BRN number:1906911
PubChem number:24886639
Physical property data
1. Character: light yellow crystal[1]
2. Melting point (℃): 51.9[2]
3. Boiling point (℃): 238.3[3]
4. Relative density (water=1): 1.29 (20℃)[4]
5. Relative vapor density (air=1): 4.72[5]
6. Saturated vapor pressure (kPa): 0.013 (20 ℃)[6]
7. Heat of combustion (kJ/mol): -3714.3[7]
8. Critical pressure (MPa): 3.8[8]
9. Octanol/water partition coefficient: 2.37[9]
10. Flash point (℃): 106 (CC) [10]
11. Ignition temperature (℃): 390[11]
12. Explosion upper limit (%): 7.6[12]
13. Explosion lower limit (%): 1.6[13] sup>
14. Solubility: Insoluble in water, easily soluble in ethanol, ether and benzene. [14]
Toxicological data
1. Acute toxicity[15]
LD50: 1960mg/kg (rat oral); >16g/kg (rat Transdermal)
2. Irritation No data available
3. Mutagenicity[16] Microbial mutagenicity: Salmonella typhimurium 10μg/dish. Microbial mutagenicity test: Salmonella typhimurium 10 μg/dish. Unprogrammed DNA synthesis: rat liver 100μg/L.
4. Carcinogenicity [17] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
Ecological data
1. Ecotoxicity[18]
LC50: 74mg/L (48h), 51mg/L (96h) (medaka) ; 73mg/L (96h) (high-bodied yarrow); 19mg/L (96h) (fathead minnow); 7.5mg/L (48h) (water flea)
EC50: 82mg/L (24h) (Tetrahymena pyriformis); 22mg/L (Single-celled green algae)
2. Biodegradability[19] MITI -I test, initial concentration 100ppm, sludge concentration 30ppm, degradation 0.8% after 2 weeks.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 37.62
2. Molar volume (cm3/mol): 117.5
3. Isotonic specific volume (90.2K ): 300.4
4. Surface tension ��dyne/cm): 42.6
5. Polarizability: 14.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 122
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[20] Stable
2. Incompatible substances[21] Strong oxidizing agent, strong reducing agent, strong alkali
3. Conditions to avoid contact[22] Heating
4. Polymerization hazard[23] No polymerization
5. Decomposition products[24] Nitrogen oxides
Storage method
Storage Precautions[25] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Add toluene to the nitrification reactor, cool it to below 25°C, add the prepared mixed acid (nitric acid 25% to 30%, sulfuric acid 55% to 58% and water 20% to 21%), increase the temperature, and adjust The temperature does not exceed 50°C. Continue to stir for 1 to 2 hours. After the reaction is completed, let it stand for 6 hours. Separate the generated nitrobenzene, wash it with water, and wash it with alkali. Crude nitrotoluene contains o-nitrotoluene and p-nitrotoluene. and m-nitrotoluene. The crude nitrotoluene is vacuum distilled to separate most of the o-nitrotoluene. The residual fraction containing more p-nitrotoluene is separated by vacuum distillation, and is cooled and crystallized to obtain p-nitrotoluene. The meta-nitrobenzene is obtained by accumulating in the mother liquor when separating the para-position body and then distilling it.
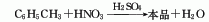
Purpose
1. Used to produce DSD acid, p-nitrobenzoic acid, p-nitrophenyl acetic acid, p-nitrotoluene orthosulfonic acid, p-methylaniline, 2,4-dinitrotoluene, 4-methyl- 1,3-phenylenediamine, 2-amino-4-chloro-5-methylbenzenesulfonic acid, 4,4′-diaminostilbene-2,2′-disulfonic acid, and 2-chloro-4- Aminotoluene, used to produce weakly acidic green GS and red base GL.
2. Used for dye synthesis. [26]

 微信扫一扫打赏
微信扫一扫打赏

