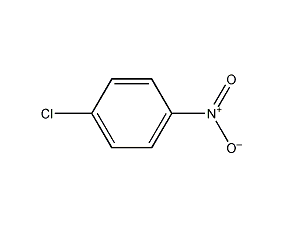
Structural formula
| Business number | 02GZ |
|---|---|
| Molecular formula | C6H4ClNO2 |
| Molecular weight | 156 |
| label |
4-Nitrochlorobenzene, 4-Nitrobenzene chloride, p-chloronitrobenzene, p-nitrochlorobenzene, 1-Chloro-4-nitrobenzene, 4-chloronitrobenzene, p-Nitrochlorobenzene, p-Nitrochlorobenzene, 1-Chloro-4-nitrobenzene, 4-Chloro-1-nitrobenzene, 4-Chloronitrobenzene, 4-Nitrochlorobenzene, p-Nitrochlorobenzene, p-Chloronitrobenzene, Pesticide intermediates; aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:100-00-5
MDL number:MFCD00007285
EINECS number:202-809-6
RTECS number:CZ1050000
BRN number:508691
PubChem number:24869528
Physical property data
1. Character: light yellow monoclinic prismatic crystal, industrial product is light yellow to light brown melted body.
2. Density (g/mL, 90/4℃): 1.2979
3. Relative vapor density (g/mL, air=1): 5.43
4. Melting point (ºC): 83~84
5. Boiling point (ºC, normal pressure): 242
6. Flash point (ºC): 127
7. Vapor pressure (kPa, 38ºC): 0.03
8. Solubility: Insoluble in water, slightly soluble in ethanol, ether, and carbon disulfide.
Toxicological data
1. Acute toxicity: rat oral LD50: 420mg/kg; rabbit dermal LD50: 16000mg/kg
2. It is a highly toxic substance. The time-weighted average allowable concentration of toxic substances in the air in the workplace is 0.6 mg/m3; the allowable concentration for short-term exposure is 1.8 mg/m3.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 37.69
2. Molar volume (cm3/mol): 113.2
3. Isotonic specific volume (90.2K ): 298.6
4. Surface tension (dyne/cm): 48.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong alkalis, and strong reducing agents. It can burn when exposed to open flames or high heat, and react with strong oxidants.
2.This product is highly toxic. Absorption through the skin or inhalation of its vapor can cause poisoning, especially when used together with ethanol, which can cause acute poisoning and death due to the formation of methemoglobin. Drinking alcohol will accelerate central nervous system and blood poisoning and cause allergies. The production workshop must have good ventilation equipment and be airtight to prevent escape, leakage, dripping and leakage. Operators must wear protective equipment.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Packed in sealed iron drums, with net weight of 200kg or 250kg per drum. Store in a cool, ventilated place. During storage and transportation, it must be protected from fire, sun and moisture. Store and transport according to regulations on toxic chemicals.
Synthesis method
After mixing nitric acid and sulfuric acid into a mixed acid, it undergoes nitration reaction with chlorobenzene to generate nitrochlorobenzene (65% para position, 34% ortho position, 1% meta position), and then separate nitrochlorobenzene and waste acid . After separation, the nitrochlorobenzene is washed with water and neutralized to obtain neutral nitrochlorobenzene, which is then dried and crystallized. The finished product p-nitrochlorobenzene is separated, and the co-product o-nitrochlorobenzene is obtained through distillation, decoking and crystallization of the co-melted oil.

Purpose
Dye intermediates. Used in the manufacture of dyes (such as azo dyes, sulfur dyes, etc.) and their intermediates (such as p-aminophenol, p-chloroaniline, etc.). It is also a raw material for medicines (such as phenacetin, paracetamol) and pesticides (parathion, herbicides).

 微信扫一扫打赏
微信扫一扫打赏

