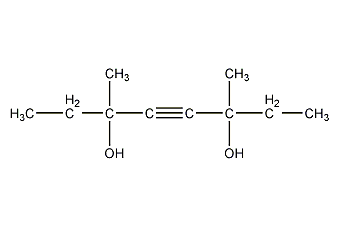
Structural formula
| Business number | 01NA |
|---|---|
| Molecular formula | C10H18O2 |
| Molecular weight | 170.25 |
| label |
3,6-dimethyl-4-octyne-3,6-diol, 3,6-dimethyl-4-octyne-3,6-diol, 3,6-Dimethyloct-4-yne-3,6-diol, 3,6-Dimethyl-3,6-dihydroxy-4-octyne, Surfynol 82 |
Numbering system
CAS number:78-66-0
MDL number:MFCD00004480
EINECS number:201-131-8
RTECS number:RI2650000
BRN number:None
PubChem ID:None
Physical property data
1. Characteristics: Crystalline solid.
2. Density (g/mL,25/4℃):0.933
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC): 53-55℃
5. Boiling point (ºC,Normal pressure):222℃
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flashpoint (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturation vapor pressure (kPa,60ºC): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (�com:office:office” />
Mouse caliber LD50: 825mg/kg;
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 49.38
2. Molar volume (m3/mol):172.0
3. isotonic specific volume (90.2K):431.8
4. Surface Tension (dyne/cm):39.7
5. Polarizability(10-24cm3):19.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 190
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
From acetylene and2- Acquired from the reaction of butanone
Purpose
Used as viscosity reducer for polyethylene plastisol, aniline Anti-gelling agent for ink, heat stabilizer for polyvinyl chloride, wetting agent for cosmetic soaps and shampoos, polyol components of polyurethane and polyester, defoaming agent for electroplating solutions, dispersants for dyes, etc.
=”FONT-SIZE: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: ‘Times New Roman’; mso-hansi-font-family: ‘Times New Roman’; mso-bidi-font-family: Arial”>(10-24cm 3):19.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 190
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
From acetylene and2- Acquired from the reaction of butanone
Purpose
Used as viscosity reducer for polyethylene plastisol, aniline Anti-gelling agent for ink, heat stabilizer for polyvinyl chloride, wetting agent for cosmetic soaps and shampoos, polyol components of polyurethane and polyester, defoaming agent for electroplating solutions, dispersants for dyes, etc.

 微信扫一扫打赏
微信扫一扫打赏

