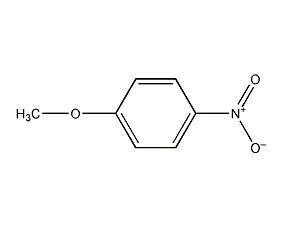
Structural formula
| Business number | 02HC |
|---|---|
| Molecular formula | C7H7NO3 |
| Molecular weight | 153.14 |
| label |
p-Nitroanisole, 4-Nitroanisole, 1-methoxy-4-nitrobenzene, p-Methoxynitrobenzene, p-nitroanisole, P-Nitroanisole, 1-Methoxy-4-nitrobenzene, 4-Nitroanisole, 4-Nitroanisole p-nitroanisole, 4-Methoxynitrobenzene, 1-Methoxy-4-nitro-benzen, 4-Methoxy-1-nitrobenzene, 4-Nitrophenyl methyl ether |
Numbering system
CAS number:100-17-4
MDL number:MFCD00007327
EINECS number:202-825-3
RTECS number:BZ8800000
BRN number:1865361
PubChem number:24846610
Physical property data
1. Properties: colorless to light yellow crystals[1]
2. Melting point (℃): 54[2]
3. Boiling point (℃): 260[3]
4. Relative density (water=1): 1.233[4]
5. Relative vapor density (air=1): 5.29[5]
6. Octanol/water partition coefficient: 2.03 [6]
7. Flash point (℃): 130[7]
8. Solubility: insoluble in water, soluble in water Compatible with most organic solvents such as ethanol and ether. [8]
Toxicological data
1. Acute toxicity: rat oral LD50: 2300mg/kg; rat skin contact LD50: >16mg/kg; rat peritoneal cavity LD50: 1400mg/kg; mouse oral LDLo: 300mg/kg; small Rat peritoneal cavity LD50: 698 mg/kg;
2. Mutagenicity: Mutant microorganism test: bacteria – Salmonella typhimurium, 6500 μmol/L; Mutant microorganism test: bacteria – Salmonella typhimurium, 2500 μg/plate; DNA repair test: Bacillus subtilis, 5mg/disc;
3. Acute toxicity[9]
LD50: 2300mg /kg (rat oral); >16g/kg (rat transdermal)
Ecological data
1. Ecotoxicity[10] LC50: 55mg/L (48h) (medaka)
2. Biodegradation Properties No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 39.47
2. Molar volume (cm3/mol): 125.2
3. Isotonic specific volume (90.2K ): 319.4
4. Surface tension (dyne/cm): 42.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.64
Computing chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 55
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances[12] Strong oxidants, strong reducing agents, strong acids, strong bases
3. Conditions to avoid contact[13] Heat
4. Polymerization hazard[14] No polymerization
5. Decomposition products[15] Nitrogen oxides
Storage method
Storage Precautions[16] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Pressurized etherification method. Obtained from the etherification of p-chloronitrobenzene and methanol.
2. Phase transfer catalysis method This method has many advantages and is suitable for a variety of reactions. Especially in nucleophilic displacement reactions, it can activate nucleophilic particles and improve their reactivity, so that many organic substances that are difficult to react can react under milder conditions, and the reaction time can be shortened and side reactions can be suppressed. . Add 0.25 mol of p-nitrochlorobenzene, 0.48 mol of methanol, and 0.0175 mol of quaternary ammonium salt phase transfer catalyst into a 250 ml three-necked flask, heat to reflux (72°C) under stirring, add 0.50 mol of sodium hydroxide at one time, and control the temperature at 80 ℃, react for 3 hours, then quench and solidify the reaction solution, wash the filter cake with cold water, dry and weigh, and take samples for gas chromatography analysis. Quaternary ammonium salts (generally preferably with a total number of carbon atoms of 12 to 15) are used as phase transfer catalysts. The material ratio is: p-nitrochlorobenzene: methanol: sodium hydroxide: catalyst = 1:1.87:2.36:0.053 (molar ratio), reaction temperature 80°C, reaction time 3h, sodium hydroxide is added at one time, p-nitrochloride The benzene conversion rate is 100%, the average yield of p-nitroanisole is 96.5%, and the freezing point is greater than 51°C.
Purpose
1. Used as dyes and pharmaceutical intermediates, mainly used in the production of p-aminoanisole, dye blue salt VB, etc.
2. Used as an intermediate in organic synthesis. [17]

 微信扫一扫打赏
微信扫一扫打赏

