Background and overview[1]
3-(3-iodo)phenylacetic acid can be used as a pharmaceutical synthesis intermediate. If 3-(3-iodo)phenylacetic acid is inhaled, move the patient to fresh air; if the skin comes in contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
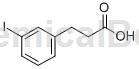
Preparation[1]
The preparation of (3-iodo)phenylacetic acid is as follows:
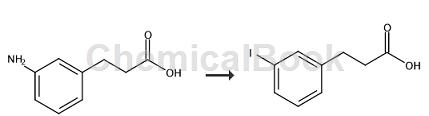
The specific steps are as follows: Stir into a stirred solution of (3-aminophenyl)propionic acid (4.91g, 29.7mmol) in water (50mL) and concentrated H2SO4 (4mL) at -7°C. Add a solution of NaNO2 (2.4g, 34.7mmol) in a minimum amount of water to the (ice-salt bath) to drain the nitrite solution below the surface of the solution and maintain the temperature below 0°C. After 10 minutes, use starch-KI indicator paper to check whether there is excess NO2- in the mixture to ensure that the diazotization is complete. Add diethyl ether (50 mL), then slowly add a solution of KI (15 g, 90 mmol) in a minimum amount of water to control the violent evolution of N2. After the addition was complete, the reaction was stirred and allowed to warm to ambient temperature over 3 hours. The layers were separated and the aqueous layer was extracted with additional diethyl ether (2 x 50 mL). The combined ether layers were back-extracted with 5% (w/v) NaHSO3 (aqueous solution), brine (1 × 25 mL), dried over MgSO4, filtered and concentrated to obtain (3-iodo)phenylacetic acid, yield 8.0 g.
1HNMR (300MHz, d6-DMSO) δ12.13 (bs, 11H), 7.62 (d, 1H, J=1.7Hz), 7.55 (dd, 1H, J=1.4,6.4Hz), 7.26 (d, 1H, J=7.4Hz), 7.09 (t, 1H, J=7.6Hz), 2.78 (t, 3H, J=7.6Hz), 2.53 (t, 3H, J=7.5Hz); MS (ESI) m/ z275(M-H).
Main reference materials
[1] U.S. Pat. Appl. Publ., 20040167188, 26 Aug 2004

 微信扫一扫打赏
微信扫一扫打赏

