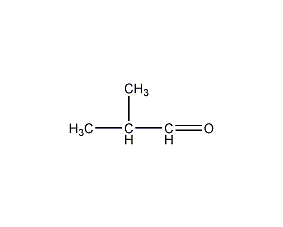
Structural formula
| Business number | 01NQ |
|---|---|
| Molecular formula | C4H8O |
| Molecular weight | 72.11 |
| label |
2-methylpropionaldehyde, dimethylacetaldehyde, Isobutyraldehyde semicarbazone, 2-Methylpropanal, Iaobutanal, 2-Methylpropionaldehyde, Aldehyde solvents, aliphatic compounds |
Numbering system
CAS number:78-84-2
MDL number:MFCD00006980
EINECS number:201-149-6
RTECS number:NQ4025000
BRN number:605330
PubChem number:24866206
Physical property data
1. Properties: colorless and transparent liquid with a strong pungent odor. [1]
2. Melting point (℃): -65[2]
3. Boiling point (℃): 64[3]
4. Relative density (water=1): 0.79 (20℃)[4]
5. Relative vapor density (air = 1): 2.48[5]
6. Saturated vapor pressure (kPa): 15.3 (20℃)[6]
7. Heat of combustion (kJ/mol): -2494.6[7]
8. Critical pressure (MPa): 4.1[8]
9. Octanol/water partition coefficient: 0.74~1.2[9]
10. Flash point (℃ ): -10.6 (OC); -40 (CC) [10]
11. Ignition temperature (℃): 196[11]
12. Explosion upper limit (%): 10.6[12]
13. Explosion lower limit (%): 1.6[13] sup>
14. Solubility: Slightly soluble in water, soluble in ethanol, ether, benzene, chloroform, carbon disulfide, acetone, toluene. [14]
15. Refractive index (n20ºC): 1.3727
16. Solubility parameter (J·cm-3)0.5: 18.235
17. van der Waals area (cm2·mol-1): 7.190× 109
18. van der Waals volume (cm3·mol-1): 49.270
19. Eccentricity factor: 0.370
20. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2500.0
21. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -247.4
22. Liquid phase standard hot melt (J·mol-1 ·K-1): 167.0
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): – 2531.5
24. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -215.8
25. Gas phase standard entropy (J·mol -1·K-1): 331.21
26. Gas phase standard free energy of formation (kJ·mol-1 ):-121.6
Toxicological data
1. Acute toxicity[15]
LD50: 960mg/kg (rat oral); 7130μl (5632.7mg)/kg (rabbit transdermal)
LC50: 39500mg/m3 (mouse inhalation, 2h)
2. Irritation [ 16] Rabbit transdermal: 397mg, mild stimulation (open stimulation test)
3. Subacute and chronic toxicity [17] Rat Link�After inhalation of 5 mg/m3 for 6 months, pathological changes occurred in the lungs; nutritional disorders occurred in various parenchymal organs, and small cell perivascular infiltration and blood vessel wall loosening occurred; the cerebral cortex swelled, and the brain Lesions of lipoprotein damage can be seen in the white matter.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[18]
Aerobic biodegradability (h): 24~168
Anaerobic biodegradability (h): 96~672
3. Non-biodegradability [19]
Half-life of photooxidation in air (h): 2.4~24
4. Other harmful effects[20] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 20.72
2. Molar volume (cm3/mol): 92.2
3. Isotonic specific volume (90.2K ): 197.5
4. Surface tension (dyne/cm): 21.0
5. Polarizability (10-24cm3):8.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 30.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: oxidized in air. Rapidly oxidizes to isobutyric acid in the presence of platinum black. The aqueous solution reacts with sodium amalgam to form isobutanol.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidizing agent, strong reducing agent, strong alkali, oxygen
4. Polymerization hazards[23] Polymerization p>
Storage method
1. Iron drums or glass bottles are packed in wooden boxes or calcium plastic boxes with reinforced inner padding. Store in a cool, ventilated warehouse. The packaging should be sealed and should not be stored for a long time to prevent deterioration. Keep away from fire and heat sources, and store and transport in isolation from oxidants.
2. Storage precautions [24] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, reducing agents, alkalis, etc. and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. The industrial method is mainly the oxo synthesis method: using propylene, carbon monoxide and hydrogen micro-raw materials, a mixture of n-butyraldehyde and isobutyraldehyde is obtained through oxo synthesis, and then the finished product is obtained through fractionation. Using different process methods, the proportions of n-butyraldehyde and isobutyraldehyde obtained are also different. The positive and negative ratio of the high-pressure method is 3-4:1, and the positive and negative ratio of the low-pressure method is 8-10:1.
Purpose
1. Isobutyraldehyde is not as widely used as n-butyraldehyde. Industrially, it is mainly produced by hydrogenation to produce isobutanol. Isobutanol can be used as a substitute for n-butanol in some aspects. The addition reaction of isobutyraldehyde and formaldehyde produces hydroxytrimethylacetaldehyde, which is then hydrogenated to form neopentyl glycol, which is used as a raw material for varnish, resin, fiber and lubricant; the fertilizer produced by the reaction of isobutyraldehyde and urea can Slowly releases nitrogen; in addition, it is also used as an intermediate for amino acids, vitamins and other drugs. The oxo synthesis method simultaneously obtains n-butyraldehyde and isobutyraldehyde in proportion. The consumption balance situation is still that the supply of isobutyraldehyde exceeds demand, and a large amount of isobutyraldehyde is used as fuel.
2. Used to synthesize cellulose esters, flavors, spices, etc., often used in baked goods, meat products, and frosting.
3. Used in the manufacture of rubber vulcanization accelerators, antioxidants, isobutyric acid, etc. [25]

 微信扫一扫打赏
微信扫一扫打赏

