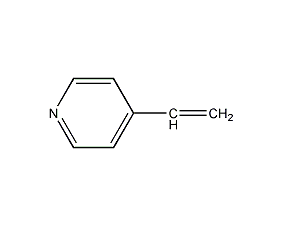
Structural formula
| Business number | 02HW |
|---|---|
| Molecular formula | C7H7N |
| Molecular weight | 105.14 |
| label |
4-vinylpyridine, 4-Vinylazobenzene, 4-vinyl azobenzene, 4-Pyridylethylene, 4-ethyridine, 4-Ethenyl-pyridin, 4-Pyridylethylene, 4-Vinyl-pyridin, Gamma-vinylpyridine, Pyridine, 4-Vinyl-, Pyridine,4-Ethenyl-, 4vp, 4-Vinylpyridine, Enzymes·Proteins·Peptides |
Numbering system
CAS number:100-43-6
MDL number:MFCD00006447
EINECS number:202-852-0
RTECS number:UU1045000
BRN number:104506
PubChem number:24900739
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 20℃): 0.988
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point ( ºC, 20KPa): 121
7. Refractive index: 1.549
8. Flash point (ºC): 52
9. Specific rotation (º) : Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water and ether , dissolve in hot water and hot ethanol.
Toxicological data
Acute toxicity: Rat oral LD50: 100mg/kg; Rat inhalation LD50: 170mg/m3; Mouse oral LC50: 161mg/kg; Mouse inhalation LC50: 380mg/m3/2H; Guinea pig oral LD50.321mg/kg; Guinea pig skin contact LDLo: 500 mg/kg; Mammalian oral LD50: 216mg/kg; Wild birds oral LD50: 100mg/kg;
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 35.26
2. Molar volume (cm3/mol): 108.6
3. Isotonic specific volume (90.2K ): 266.3
4. Surface tension (dyne/cm): 36.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 12.9
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 72.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid exposure to light, strong oxidants, and acids.
Storage method
1. Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The storage temperature should not exceed 30℃. Keep container sealed and strictly prohibited from contact with air. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Not suitable for large amounts 2. Storage or long-term storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of 4-methylpyridine and formaldehyde to generate 4-pyridine ethanol, and then dehydration. The reaction formula is as follows:
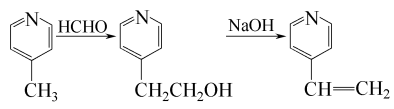
The above process is the process method currently mainly used in foreign industries, such as Japan’s Guangei Chemical Industry Company.
Add 3720kg of 4-methylpyridine, 304kg of paraformaldehyde, 61kg of benzoic acid, and 180kg of distilled water into the three-port enamel reactor. Heat and reflux under normal pressure for 60 hours. The boiling temperature will be 105°C at the beginning and the boiling temperature at the end. Can be above 120℃. First, unreacted 4-methylpyridine is evaporated under reduced pressure, and then all unreacted 4-methylpyridine is evaporated under reduced pressure of 13.3KPa to obtain 498kg of crude pyridine ethanol. The catalyst benzoic acid was in the recovered methylpyridine, and a total of 3750kg of recovered liquid (water, methylpyridine, benzoic acid, formaldehyde) was obtained. Then add 385kg of 4-methylpyridine and 110kg of paraformaldehyde to carry out the second reaction. Carry out 8 reactions in this manner to obtain a total of 4000kg of pyridine ethanol, consuming 3800kg of 4-methylpyridine and 310kg of paraformaldehyde, with a yield of 95%. Pyridine ethanol dehydration operation procedure: Put 100kg of 40% to 50% industrial sodium hydroxide aqueous solution into a three-mouth reaction kettle, add 0.1kg of methylene blue, raise the temperature to 140 to 190°C, and evacuate to (600 to 700) × 133.3Pa. Begin to dropwise add pyridine ethanol containing 0.1% methylene blue. Vinylpyridine and water are immediately distilled out, quickly cool to room temperature, and add 0.1% methylene blue, and add 5% (mass fraction )Solid industrial sodium hydroxide, let it sit for 24 hours, the solution will be divided into two layers. The upper layer is vinylpyridine. Add 0.1% methylene blue for vacuum distillation. The boiling point must be below 50°C to avoid polymerization. Add 0.1% methylene blue to pure 4-vinylpyridine to prevent polymerization. It can be left for more than one year. The yield is above 80%.
Purpose
1. Used as monomers for organic synthesis intermediates and polymers. Used in functional polymers, surfactants, antistatic agents, photosensitive resins, coatings, medicines, pesticides and many other aspects.
2.After adding this product to synthetic resins such as vinyl chloride, the mechanical strength and bending strength of the resin can be greatly enhanced. As an important monomer for the synthesis of high molecular polymers, this product can perform solution polymerization, emulsion polymerization, suspension polymerization, etc. in the presence of heat, light, and initiators such as benzoyl oxide. The copolymer film made of this product and methacrylate has good antioxidant properties. It reacts with divinylbenzene to obtain a bridge polymer. Its ammonium salt has ion exchange properties, can easily remove heavy metals, absorb organic acids, and can also be used as a catalyst for dehydrochlorination reaction.

 微信扫一扫打赏
微信扫一扫打赏

