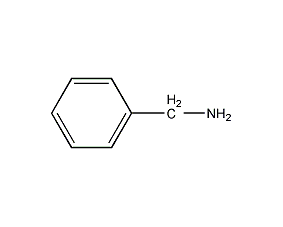
Structural formula
| Business number | 02HZ |
|---|---|
| Molecular formula | C7H9N |
| Molecular weight | 107.15 |
| label |
benzylamine, Alpha-aminotoluene, Benzylamine, Phenylmethylamine, N-Benzylamine, Benzenemethaneamine, Monobenzylamine, Nitrogen-containing compound solvents, Precipitant, aromatic compounds |
Numbering system
CAS number:100-46-9
MDL number:MFCD00008106
EINECS number:202-854-1
RTECS number:DP1488500
BRN number:741984
PubChem ID:None
Physical property data
1. Properties: light amber liquid.
2. Density (g/mL, 20/4℃): 0.9813
3. Melting point (ºC): 10
4. Boiling point (ºC, Normal pressure): 185
5. Boiling point (ºC, 1.60KPa): 90
6. Refractive index (20ºC): 1.5401
7. Flash point (ºC): 60
8. Vapor pressure (kPa, 90ºC): 1.60
9. Heat of combustion (KJ/mol): 4052.1
10. Solubility: Miscible with water, ethanol and ether, soluble in acetone and benzene, slightly soluble in chloroform.
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD50: 600mg/kg; mammalian oral LD50: 700mg/kg;
2. Oral administration is toxic. It is corrosive and can cause burns.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 34.70
2. Molar volume (cm3/mol): 109.4
3. Isotonic specific volume (90.2K ): 273.1
4. Surface tension (dyne/cm): 38.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 55.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with acids, acid chlorides, acid anhydrides, strong oxidants, and carbon dioxide. It is corrosive and protective clothing and gloves should be worn when using it. It is alkaline and can absorb carbon dioxide.
2. Exist in mainstream smoke.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The preparation method is to add ethanol, urotropine and benzyl chloride into a reaction kettle, heat to 30-35°C, react for 4 hours, add hydrochloric acid, heat to 45-50°C, react for 2 hours, cool, filter, and heat the filtrate Remove ethanol, change to distillation under reduced pressure, steam until almost dry, add alkali solution, free benzylamine, distill under normal pressure and then distill under reduced pressure to obtain the product.
You can also use the ammonolysis method to add benzyl chloride, ammonium hydroxide, and ammonium bicarbonate into the reaction pot, react at 30 to 35°C for 6 hours, let it stand to separate the oil layer, raise the temperature of the reaction solution to catch the ammonia, and distill under reduced pressure at 100°C. , then add free alkali, separate the alkali solution, and distill the oil layer to obtain the product.
Purpose
Precipitating agent for the determination of molybdate, barate, tungstate, thorium, zirconium, cerium, lanthanum, praseodymium and neodymium in microcrystalline analysis. Used in organic analysis to distinguish various types of carboxylic acids (react with carboxylic acid esters to generate N-benzylamine derivatives, and distinguish various carboxylic acids based on different melting points). Qualitative testing of organometallic compounds. Also used as solvent and organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

