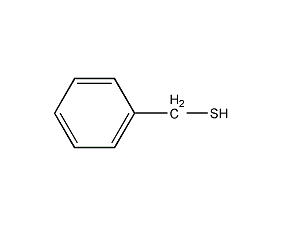
Structural formula
| Business number | 02J5 |
|---|---|
| Molecular formula | C7H8S |
| Molecular weight | 124.2 |
| label |
benzyl mercaptan, benzylthiol, α-Toluenethiol, benzyl mercaptan, Toluenethiol, Benylmercaptan, benzyl hydrosulfide, Alpha-toluenethiol, A-Toluenethiol, Benzyl Mercaptan, Benzenemethanethiol, Fema 2147, Alpha-Toluenethiol, Alpha-Tolyl Mercaptan, Toluenethiol, Toluene-A-Thiol, aromatic sulfur compounds |
Numbering system
CAS number:100-53-8
MDL number:MFCD00004867
EINECS number:202-862-5
RTECS number:XT8650000
BRN number:605864
PubChem number:24848079
Physical property data
1. Properties: water-white liquid with strong smell. [1]
2. Melting point (℃): -29.05[2]
3. Boiling point (℃): 194.5[3]
4. Relative density (water = 1): 1.06[4]
5. Relative vapor Density (air=1): 4.28[5]
6. Critical pressure (MPa): 4.06[6]
7. Octanol/water partition coefficient: 2.480[7]
8. Flash point (℃): 70[8]
9. Explosion upper limit (%): No data yet
10. Explosion lower limit (%): 1.1[9]
11. Solubility: insoluble in water, soluble in ethanol, ether, and carbon disulfide. [10]
Toxicological data
1. Acute toxicity[11]
LD50: 493mg/kg (rat oral)
LC50 :>235ppm (rat inhalation, 4h); 902mg/m3 (mice inhalation, 4h)
2. Irritation No data available yet
Ecological data
1. Ecotoxicity[12] EC50: 0.26mg/L (24h) (Daphnia, static)
2 .Biodegradability No data yet
3. Non-biodegradability[13] In the air , when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 9h (theoretical).
4. Bioaccumulation [14] BCF: 16 (theoretical)
5. Other harmful Effect[15] This substance is hazardous to the environment.�, attention should be paid to the pollution of the water environment and aquifers.
Molecular structure data
1. Molar refractive index: 39.06
2. Molar volume (cm3/mol): 119.7
3. Isotonic specific volume (90.2K ): 295.9
4. Surface tension (dyne/cm): 37.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 55.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances[17] Strong oxidants, strong alkalis
3. Conditions to avoid contact[18] Heating
4. Polymerization hazard[19] No polymerization
5. Decomposition products[20] Hydrogen sulfide
Storage method
1. Storage precautions [21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Store tightly in a glass bottle, covered with an iron drum. Store in a cool, dry, ventilated place, protected from sun and moisture, and away from fire and heat sources. Store and transport according to regulations on irritating items.
Synthesis method
Put S-benzylisothiourea hydrochloride and 10% sodium hydroxide solution into the reactor, heat under direct fire and reflux for 2 hours to carry out hydrolysis. Then cool, acidify with dilute sulfuric acid, and extract with diethyl ether. The extract is dried with anhydrous sodium sulfate, then the desiccant is filtered off, and the diethyl ether is recovered. The residue is distilled under reduced pressure, and the fractions between 98 and 108°C (2.6664kPa) are collected. , that is, the finished product is obtained.
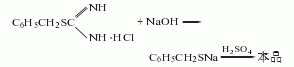
Purpose
1. Used in flavor manufacturing. [22]

 微信扫一扫打赏
微信扫一扫打赏

