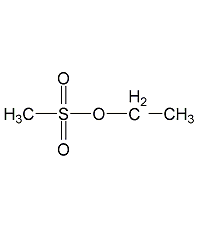
structural formula
| business number | 01cc |
|---|---|
| molecular formula | c3h8o3s |
| molecular weight | 124.16 |
| label |
ethyl methanesulfonate, ethyl mesylate, methanesulfonic acid ethyl ester, ch3so3ch2ch3 |
numbering system
cas number:62-50-0
mdl number:mfcd00007559
einecs number:200-536-7
rtecs number:pb2100000
brn number:773969
pubchem number:24896575
physical property data
1. appearance: colorless transparent oily liquid
2. density (g/ml, 20/4℃): 1.206
3. relative vapor density (g/ml , air=1): uncertain
4. melting point (ºc): uncertain
5. boiling point (ºc, normal pressure): uncertain
6. boiling point (ºc, 10mmhg): 85-86(lit.)
7. refractive index: n20/d 1.418(lit.)
8. flash point (ºc): 100
9. specific rotation (º): uncertain
10. autoignition point or ignition temperature (ºc ): uncertain
11. vapor pressure (kpa, 25ºc): uncertain
12. saturated vapor pressure (kpa, 60ºc): uncertain
13. heat of combustion (kj/mol): uncertain
14. critical temperature (ºc): uncertain
15. critical pressure (kpa): uncertain
16. the logarithmic value of the oil-water (octanol/water) partition coefficient: uncertain
17. the upper limit of explosion (%, v/v): uncertain
18. lower explosion limit (%, v/v): uncertain
19. solubility: soluble in ether, ethanol, chloroform, slightly soluble in water.
toxicological data
none yet
ecological data
none yet
molecular structure data
1. molar refractive index: 26.48
2. molar volume (cm3/mol): 105.4
3. isotonic specific volume (90.2k): 254.3
4. surface tension (dyne/cm): 33.8
5. polarizability (10-24cm3): 10.50
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 0
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: none
6. topological molecule polar surface area 51.8
7. number of heavy atoms: 7
8. surface charge: 0
9. complexity: 118
10. number of isotope atoms: 0
11.determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be sealed and stored in a cool, dry place.
synthesis method
1. the methylsulfonyl chloride obtained by reacting dimethyl sulfate with sodium thiosulfate is esterified with ethanol to obtain ethyl methanesulfonate.
2. preparation method:
mix methanesulfonyl chloride (2) and absolute ethanol evenly, cool to 0°c, slowly add pyridine dropwise while stirring, and control the temperature of the reaction solution not exceeding 10℃. after addition, acidify with hydrochloric acid and extract twice with diethyl ether. the ether layer was washed with water, washed with 5% sodium carbonate solution to ph 7-8, and dried over anhydrous sodium sulfate. after distilling off the ether, distill under reduced pressure and collect the fraction at 85-86°c/1.33kpa to obtain compound (1). [1]
purpose
used as a bacterial mutagen, as well as in organic synthesis and cancer research.

 微信扫一扫打赏
微信扫一扫打赏

