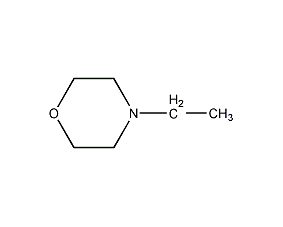
Structural formula
| Business number | 02JM |
|---|---|
| Molecular formula | C6H13NO |
| Molecular weight | 115.18 |
| label |
4-Ethylmorpholine, N-Ethylmorpholine, N-Ethyltetrahydro-1,4-oxazine, Ethyl p-oxazine, Ethylmorpholine, N-Ethylmorpholine, 4-Ethyl-Morpholine, Ethylmorpholine, N-Ethylmorpholine, N-Ethyldiethylenimideoxide, N-Ethylmorfoline, N-Ethyltetrahydro-1,4-Oxazine, rubber vulcanization accelerator, Catalyst for copolymerization, Catalysts for the manufacture of urethane polymers, Multifunctional solvent |
Numbering system
CAS number:100-74-3
MDL number:MFCD00006177
EINECS number:202-885-0
RTECS number:QE4025000
BRN number:102969
PubChem ID:None
Physical property data
1. Properties: colorless liquid
2. Relative density (g/mL, 20/20℃): 0.916
3. Density (g/mL, 20/ 4℃): 0.8996
4. Relative vapor density (g/mL, air=1): 4.00
5. Melting point (ºC): -63
6. Boiling point (ºC, normal pressure): 138
7. Refractive index (20ºC): 1.4400
8. Flash point (ºC): 32
9. Vapor pressure (kPa): 0.813
10. Solubility: miscible with water, ethanol, and ether. Soluble in acetone and benzene.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 453mgREACTION SEVERITY, slight reaction; 2. Acute toxicity: rat oral LD50: 1780mg/kg; rat inhalation LCLo: 2000ppm/4H; mouse oral LD50: 1200mg/kg; Mouse inhalation LC50: 18000mg/m3/2H; Mouse intravenous injection LD50: 180mg/kg; Mammalian oral LD50: 1200mg/kg; 3. Mutagenicity: Mutant microbial test: Bacteria-mouse Salmonella typhi, 6667μg/plate; 4. Irritating to skin and eyes. The maximum allowable concentration in the workplace is 94mg/m3.
Ecological data
The substanceWater is slightly harmful.
Molecular structure data
1. Molar refractive index: 32.94
2. Molar volume (cm3/mol): 126.1
3. Isotonic specific volume (90.2K ): 287.3
4. Surface tension (dyne/cm): 26.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 12.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 59.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides. This product is a relatively stable compound, but easy to burn. Has the chemical properties of a tertiary amine (pKa 7.7).
2. This product can irritate the skin and mucous membranes like morpholine, but is less toxic and does not cause changes in the liver and kidneys. Mice inhaled LC5018mg/L. Rats were given LD501.2g/kg by gavage. According to changes in the total capacity of the central nervous system, the threshold concentration of this product for one action is 0.09 ~ 0.1mg/L. Repeated administration and gradual increase in concentration will lead to habituation. The threshold concentration for human irritation is 0.22 mg/L. The irritating effect on skin is 1/20 of morpholine. The allowable concentration in air is 5 mg/m3. For personal protection and precautions see Morpholine (D059).
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. Store tightly in glass bottles or ceramic jars and package in wooden barrels. Store in a cool, dry and ventilated place. Protect from sun and moisture, and keep away from fire and heat sources. When transporting, load and unload gently to avoid damage to the packaging. Store and transport according to regulations on toxic chemicals.
Synthesis method
Derived from the reaction of morpholine and ethyl bromide.
Refining method: After distilling twice, pass in hydrogen chloride gas to convert it into hydrochloride (very easy to absorb moisture), and then recrystallize it with a mixture of absolute ethanol and acetone (1:2).
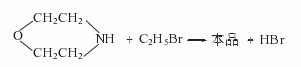
Purpose
Used as a solvent for grease, dyes, resins, etc. It is also used as rubber vulcanization accelerator, catalyst for butadiene polymerization and copolymerization, catalyst for urethane polymer production, etc. In addition, N-ethylmorpholine can also be used as a synthetic intermediate for dyes, medicines, surfactants, etc.
Used as solvent and raw material for organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

