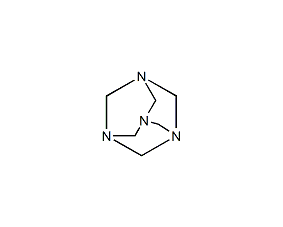
Structural formula
| Business number | 02JX |
|---|---|
| Molecular formula | C6H12N4 |
| Molecular weight | 140.19 |
| label |
1,3,5,7-tetraazatricyclo[3.3.1.13]decane, amine imitation, accelerator H, Heikesha, Hexamethylenetetramine, Hexagonal methenamine, hexamethyleneimine, Oritropine, 1,3,5,7 – Tetraaza ring [3.3.1.13] decane-, Amine imitation, promoter H, Hai Kesha, hexamethylenetetramine, Hai Kesha hexamine, 6 methylene Kia amine, Hardener, Organic corrosion inhibitor materials |
Numbering system
CAS number:100-97-0
MDL number:MFCD00006895
EINECS number:202-905-8
RTECS number:MN4725000
BRN number:2018
PubChem number:24895743
Physical property data
1. Characteristics: White fine granular crystals, tastes sweet at first and then bitter. [1]
2. pH value: 8.4 (0.2mol/L aqueous solution) [2]
3. Melting point (℃): 280~295 (decomposition) [3]
4. Boiling point (℃): 263 (sublimation) [4]
5. Relative density (water=1): 1.33[5]
6. Relative vapor density (air=1): 4.9[6 ]
7. Heat of combustion (kJ/mol): -239.7[7]
8. Critical pressure (MPa): 3.68 [8]
9. Octanol/water partition coefficient: -4.15[9]
10. Flash point ( ℃): 250[10]
11. Solubility: soluble in water, ethanol, chloroform, carbon tetrachloride, insoluble in ether, petroleum ether, and aromatic hydrocarbons. [11]
Toxicological data
1. Acute toxicity[12] LD50: 9200mg/kg (rat intravenous); 569mg/kg (mouse oral )
2. Irritation No information available
Ecological data
1. This substance is slightly harmful to water. Irritates the skin and can cause dermatitis and skin eczema. Staff should take good precautions. If they accidentally touch their skin or eyes, they should rinse immediately with plenty of running water.
2. Ecotoxicity No information available
3. Biodegradability[13] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, 22% degradation after 2 weeks.
4. Non-biodegradability<str��Tetramine. Or it can be produced by the mixed reaction of nitrogen and formaldehyde solution.
4. A condensation reaction occurs between formaldehyde and excess ammonia under certain conditions. After the reaction is completed, it is settled, filtered, and the filtrate is vacuum evaporated to a paste, filtered and dried.
5. Mix and evaporate the formaldehyde aqueous solution and ammonia water to obtain hexamethylenetetramine. Or it can be produced by mixing nitrogen and formaldehyde solution.
Purpose
1. Used as a curing agent for resins and plastics, a vulcanization accelerator for rubber, an anti-shrinkage agent for textiles, and used to make fungicides, explosives, etc. Hexamethylenetetramine is mainly used as a curing agent for resins and plastics, a catalyst and foaming agent for aminoplastics, an accelerator for rubber vulcanization (accelerator H), and an anti-shrinkage agent for textiles. Hexamethylenetetramine is a raw material for organic synthesis and is used in the pharmaceutical industry to produce chloramphenicol. Hexamethylenetetramine can be used as a disinfectant in the urinary system. It has no antibacterial effect and is effective against Gram-negative bacteria. Its 20% solution can be used to treat underarm odor, sweaty feet, tinea corporis, etc. Mixed with sodium hydroxide and sodium phenolate, it can be used as a phosgene absorber in gas masks. and used in the manufacture of pesticides and insecticides. The interaction between hexamethylenetetramine and fuming nitric acid can produce a highly explosive cyclone explosive, referred to as RDX. Hexamethylenetetramine can also be used as a reagent and chromatographic analysis reagent for the determination of bismuth, indium, manganese, cobalt, thorium, platinum, magnesium, lithium, copper, uranium, beryllium, tellurium, bromide, iodide, etc.
2. One of the commonly used corrosion inhibitors for hydrochloric acid pickling of large boilers. It can be well adsorbed on the metal surface and form a protective film to inhibit the corrosive effect of hydrochloric acid on the metal. When used as a corrosion inhibitor for hydrochloric acid pickling, the dosage alone is 0.5%.
3. Used as analytical reagents, such as preparing buffer solutions and as masking agents. Also used as vulcanization accelerator.
4. It is mainly used as a pH regulator for the synthesis of water-soluble amino resins, and can also be used as a curing agent for phenolic resins, rubber vulcanization accelerators, plastic foaming agents, aqueous explosive sensitizers, etc. In the textile industry, it is used as a buffering agent for fabric anti-shrinkage finishing agent and fabric waterproof finishing agent CR to prevent cotton fabrics from being brittle and damaged. Because it can decompose to form formaldehyde in acidic media, it will be oxidized to form acid during the bleaching process, reducing the pH value to about 4, so it can be used as a sodium chlorite bleaching activator. Because of its disinfecting and diuretic effects, it is used medicinally as a diuretic and disinfectant. Used as protein hardening agent and preservative in food and cosmetics industry. Mixed with caustic soda and sodium phenolate, it can be used as a phosgene absorbent for gas masks. It can also be used as a hide preservative, latex and pigment protective agent, lubricating oil stabilizer, catalyst and intermediate for the manufacture of pesticides. It is also an analytical reagent for the determination of copper, iron, magnesium, manganese, cobalt, thorium, platinum, indium, etc.
5. Mix with caustic soda and sodium phenolate and used as phosgene absorbent in gas masks. It is also used as a buffering agent for sodium hypochlorite bleach activator and waterproofing agent CR. It is also used as a fungicide and as a deodorant and insecticide.
6. Used as anti-shrinkage finishing agent for textiles, sodium chlorate bleach activator, buffering agent for waterproofing agent CR, etc. [21]

 微信扫一扫打赏
微信扫一扫打赏

