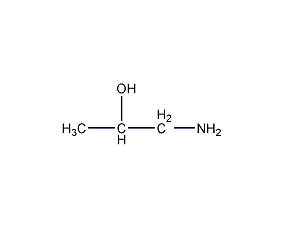
Structural formula
| Business number | 01P0 |
|---|---|
| Molecular formula | C3H9NO |
| Molecular weight | 75.11 |
| label |
(±)-isopropanolamine, 3-amino-2-propanol, 1-Aminoisopropanol, 2-hydroxy-n-propylamine, (±)-Isopropanolamine, DL-Isopropanolamine, 1-Amino-iso-proanol, iso-Propanolamine, 2-Hydroxy-n-propylamine, iso-Propanolamine, Multifunctional solvents, Surfactant, plasticizer, emulsifier, Antistatic agents for the fiber industry |
Numbering system
CAS number:78-96-6
MDL number:MFCD00008139
EINECS number:201-162-7
RTECS number:UA5775000
BRN number:605275
PubChem number:24846971
Physical property data
1. Properties: Colorless or slightly yellow liquid. Slightly ammonia smell.
2. Density (g/mL, 20/20℃): 0.9681
3. Melting point (ºC): 1.4
4. Boiling point (ºC, Normal pressure): 159.4
5. Refractive index (20ºC): 1.4479
6. Flash point (ºC): 73.89
7. Viscosity (mPa· s, 20ºC): 31
8. Solubility: Miscible with water and ethanol, insoluble in ether.
Toxicological data
1. Acute toxicity: rat caliber LD50: 1715mg/kg; rat abdominal cavity LD50: 250 mg/kg
Rabbit skin LD50: 1640 uL/kg; feeding animal LD50: >1mg/kg
2. Neurotoxicity: Rabbit skin test: 485mgREACTION SEVERITY
Rabbit skin test: 500 mg/24HREACTION SEVERITY
Rabbit eye test: 970ug/REACTION SEVERITY
p; Rabbit eye test: 250ug/24HREACTION SEVERITY
3. It is irritating to the eyes, mucous membranes and skin, and can cause burns in severe cases.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 20.97
2. Molar volume (cm3/mol): 79.6
3. Isotonic specific volume (90.2K ): 194.6
4. Surface tension (dyne/cm): 35.7
5. Polarizability (10-24cm3): 8.31
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Topological molecular polar surface area (TPSA): 46.3
6. Number of heavy atoms: 5
7. Surface charge: 0
8. Complexity: 22.9
9. Number of isotope atoms: 0
10. Determine the atomic stereocenter Number: 0
11. Number of uncertain atomic stereocenters: 1
12. Number of determined chemical bond stereocenters: 0
13. Uncertain chemical bonds Number of stereocenters: 0
14, Number of covalent bond units: 1
Properties and stability
1. Isopropanolamine is not particularly toxic because it contains amino groups and is harmful to the eyes and skin. There is some degree of damage. For rats, the oral toxicity LD50 is 4.26g/kg. The LD50 for humans is 4.3g/kg.
2. It is corrosive and can cause burns , avoid inhaling the vapor and fumes of this product.
Storage method
1. This product should be kept sealed.
Packed in iron drums, 100kg per drum, and 200kg drums are also available abroad. Pay attention to fire and heat protection during storage and transportation.
Synthesis method
The main uses of 1-amino-2-propanol (isopropanolamine) are as follows: (1) The reaction with fatty acids produces fatty acid isopropanol amides (alkyl isopropanol amides) and esters. Due to its excellent Due to its foaming properties, foam stability and ability to dissolve grease, it can be used as an industrial synthetic detergent. (2) The product obtained from thioglycolic acid can be used as a base for cosmetics. (3) Due to its good hygroscopicity and weak alkalinity, it can be used as a raw material for surfactants, and is used as a refining agent, antistatic agent, dyeing auxiliary and fiber wetting agent in the fiber industry. (4) Its phosphates and nitrites can be used as antioxidants in various lubricants and cutting oils. (5) The reaction products with various acids and higher fatty ketones can be used as plasticizers, emulsifiers and solvents. The isopropanolamine mixture has a particularly strong ability to dissolve hydrocarbons. Stir kerosene, halogenated hydrocarbons, naphtha, etc. with isopropanolamine (4%) and oleic acid (15%) in water to obtain a stable emulsion. . The salt formed with long-chain fatty acids can be used as an emulsifier for vinyl acetate resin. The emulsion has good stability and stable color.
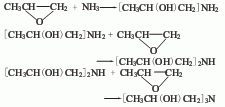
It can be produced by the reaction of propylene oxide and ammonia. After mixing propylene oxide and ammonia, the addition reaction is carried out through preheating, and the resulting mixture is deaminated, dehydrated, distilled under reduced pressure, and rectified to obtain the finished product. If the feeding ratio of propylene oxide and ammonia is adjusted during the reaction, different proportions of isopropanolamine, diisopropanolamine and triisopropanolamine can be produced.
Purpose
1. Used as solvent, surfactant, plasticizer, emulsifier, and antistatic agent in fiber industry. Rubber vulcanization accelerator.
2.Used as surfactant raw materials and detergents, it can be used to prepare non-water-soluble detergents, such as dry cleaning products, etc. .

 微信扫一扫打赏
微信扫一扫打赏

