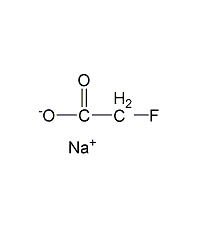
Structural formula
| Business number | 01CL |
|---|---|
| Molecular formula | C2H2FNaO2 |
| Molecular weight | 100.02 |
| label |
Fluoroacetic acid sodium salt, Fluoroacetic acid sodium salt, Gifblaar poison |
Numbering system
CAS number:62-74-8
MDL number:MFCD00002682
EINECS number:200-548-2
RTECS number:AH9100000
BRN number:3915223
PubChem number:24862768
Physical property data
1. Character:White powder, almost odorless. Hygroscopic. Not volatile.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):200 -205(dec.)
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (kPa,60ºC): Unsure
13. Heat of combustion (KJ/mol): Unsure
14. Critical temperature (ºC): Unsure
15. Critical pressure (KPa): Unsure
16. Oil and water (octanol/Log value of water) partition coefficient: Uncertain
17. Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:Easily soluble in water, insoluble in various organic solvents.
Toxicological data
Highly toxic, lethal dose (orally) is about 2~5mg/kg. Corrosive.
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 40.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 46.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Industrial products have a faint smell of acetate. It easily absorbs moisture and becomes sticky in the air. Acute oral LD50 (mg/kg): 0.22 (Rattus norvegicus), 8.0 (Mus musculus), 0.65 (Long-clawed gerbil), 0.06 (dog), poison bait concentration 0.1% ~ 0.3%. Because it is too toxic to humans and animals, has rapid onset of potency, and has secondary toxicity, China has banned its production and ban.
Storage method
Save at room temperature, away from light and heat ; Keep in a well-ventilated and dry place and store in a sealed container; avoid contact with eyes, skin, clothing, and avoid prolonged or repeated exposure.
Synthesis method
It is produced by the pressure reaction of methyl chloroacetate and hydrogen fluoride to form methyl fluoroacetate, and then alkaline hydrolysis.
Purpose
Fluoride synthesis.
mes New Roman'”> Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:Easily soluble in water, insoluble in various organic solvents.
Toxicological data
Highly toxic, lethal dose (orally) is about 2~5mg/kg. Corrosive.
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 40.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 46.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Industrial products have a faint smell of acetate. It easily absorbs moisture and becomes sticky in the air. Acute oral LD50 (mg/kg): 0.22 (Rattus norvegicus), 8.0 (Mus musculus), 0.65 (Long-clawed gerbil), 0.06 (dog), poison bait concentration 0.1% ~ 0.3%. Because it is too toxic to humans and animals, has rapid onset of potency, and has secondary toxicity, China has banned its production and ban.
Storage method
Save at room temperature, away from light and heat ; Keep in a well-ventilated and dry place and store in a sealed container; avoid contact with eyes, skin, clothing, and avoid prolonged or repeated exposure.
Synthesis method
It is produced by the pressure reaction of methyl chloroacetate and hydrogen fluoride to form methyl fluoroacetate, and then alkaline hydrolysis.
Purpose
Fluoride synthesis.
h2 id=”yt”>Purpose
Fluoride synthesis.

 微信扫一扫打赏
微信扫一扫打赏

