Background and overview[1]
4-Nitro-3,5-dimethylbenzoic acid is an organic intermediate that can be obtained by oxidation of 1,3,5-2-nitrobenzene.
Preparation[1]
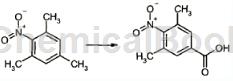
To an acetic acid solution in which chromium (VI) oxide (10.0g) is dissolved, an acetic acid solution of 1,3,5-trimethyl-2-nitrobenzene (5.00g) is added dropwise at 70°C. After the dropwise addition is completed, the mixture is heated and stirred at the same temperature for 30 minutes. Isopropyl alcohol (11 ml) was added thereto, and the mixture was stirred with heating at 50°C for 30 minutes. Pour water into the solution and cool it in an ice bath. The crystals thus obtained were filtered and taken out, dissolved in ethyl acetate, and dried over magnesium sulfate. After separation of the desiccant by filtration, the solvent was evaporated under reduced pressure to obtain a crude product. The obtained crude product was washed with hexane to obtain 3,5-dimethyl-4-nitrobenzoic acid (1.40 g). 1H-NMR (CDCl3) δ : 2.37 (3H, s), 7.89 (IH, s).
Apply[1]
Can be used to prepare 4-nitro-3,5-dimethylbenzyl alcohol:
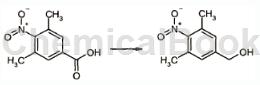
Dissolve 3,5-dimethyl-4-nitrobenzoic acid (1.39g) in THF under an argon atmosphere. Under ice cooling, a 0.9N diborane THF solution was added dropwise thereto. After completion of the dropwise addition, the mixture was stirred at the same temperature for 30 minutes. The mixture was then returned to room temperature and stirred again overnight. A mixed solvent containing water and THF was slowly added under ice cooling until foaming stopped. The mixture was then poured onto water and extracted twice with ethyl acetate. The organic layers were combined and dried over magnesium sulfate. After the desiccant was separated by filtration, the solvent was evaporated under reduced pressure to obtain (3,5-dimethyl-4-nitrophenyl)methanol (117 g). 1H-NMR (CDCl3) δ: 1.79 (1H, t), 2.32 (3H, s), 4.68 (1H, d), 7.13 (1H, s ).
Main reference materials
[1] From PCT Int. Appl., 2010015355, 11 Feb 2010

 微信扫一扫打赏
微信扫一扫打赏

