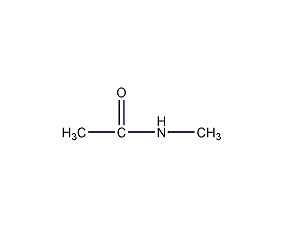
Structural formula
| Business number | 01PF |
|---|---|
| Molecular formula | C3H7NO |
| Molecular weight | 73.09 |
| label |
acetylmethylamine, Acetylmethylamine, deacidifying agent, Nitrogen-containing compound solvent |
Numbering system
CAS number:79-16-3
MDL number:MFCD00008683
EINECS number:201-182-6
RTECS number:AC5960000
BRN number:1071255
PubChem number:24884026
Physical property data
1. Properties: White needle-like crystals
2. Density (g/mL, 25/4℃): 0.9371
3. Melting point (ºC): 26~28
4. Boiling point (ºC, normal pressure): 204~206
5. Boiling point (ºC, 12kPa): 140.5
6. Refractive index (28ºC ): 1.4301
7. Viscosity (mPa·s, 35ºC): 3.23
8. Flash point (ºC): 108
9. Heat of evaporation ( KJ/mol, 115~205ºC): 59.5
10. Heat of fusion (J/mol): 8.37
11. Critical temperature (ºC): 417
12. Conductivity (S/m, 40ºC): 2×10-1
13. Vapor pressure (kPa, 56ºC): 0.2
14. Solubility: Easily soluble in water, alcohol, ether, acetone, benzene, chloroform, insoluble in solvent gasoline.
Toxicological data
The oral LD50 in rats is 3200 mg/kg. The LD50 of intravenous injection in mice is 4010 mg/kg. The LD50 of intraperitoneal injection in mice was 4380 mg/kg. Rats on the 13th day of gestation were given 750 mg/kg of N-methylacetamide and developed fetal abnormalities.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 19.57
2. Molar volume (cm3/mol): 83.9
3. Isotonic specific volume (90.2K ): 184.5
4. Surface tension (dyne/cm): 23.2
5. Polarizability (10-24cm3): 7.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 42.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Sex� and stability
1. Chemical properties: It reacts with hydrogen chloride to produce two forms of salts, namely CH3CONHCH3·HCl (melting point 67.2~69.4℃) and (CH3CONHCH3)2·HCl. It hardly reacts with metallic sodium at room temperature. Reacts with nitrous acid to form nitroso compounds. Boiling under reflux with acetic anhydride generates N-methyldiethylamide. Under the action of acid or alkali, N-methylacetamide can be hydrolyzed.
2. Exist in smoke.
Storage method
This product should be sealed and stored in a cool, dark place. Store in a dry and dark place away from wind and light.
Synthesis method
Obtained from the reaction of ethyl acetate and methylamine. Mix ethyl acetate and 65% methylamine, heat to about 60°C, and react for 4 days and nights until there is no more stratification, that is, the reaction is completed. Recover ethanol by distillation under reduced pressure, and collect the 95-110°C (4.0kPa) fraction to obtain N-methylacetamide.
Refining method: N-methylacetamide is synthesized by the reaction of methylamine and acetic acid. Generally, it is refined by multiple fractional distillation and fractional crystallization, and a product with a conductivity of 5×10-8 S/m can be obtained. N-methylacetamide used for studying the conductivity of salts can be shaken by adding phosphorus pentoxide, filtered with glass wool and then vacuum distilled. Repeat this operation three times and then distill it twice to obtain a pure product with a conductivity of 4.2×10-7S/m.
Purpose
1. Because it has excellent properties of dissolving other organic matter, it is often used as a solvent in organic synthesis. It is widely used in the pharmaceutical industry, such as the synthesis of cephalosporins, and it is used as a solvent. N-methylacetamide has a catalytic effect on certain chemical reactions and is a deacidifying agent in non-polar solvents, so it is currently widely used.
2. Used as pesticide intermediates.

 微信扫一扫打赏
微信扫一扫打赏

