Background and overview[1]
4-tert-butyl-2,6-dinitrophenol is an organic intermediate that can be prepared by nitration of 4-tert-butylphenol.
Preparation[1]
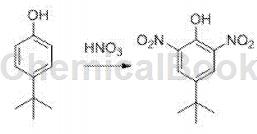
Dissolve 4-tert-butylphenol (25g, 170mmol) in 100mL glacial acetic acid, and place it in an ice environment. Add a mixture of concentrated nitric acid (50mL) and glacial acetic acid (75mL) in batches, and control the dripping time More than 45 minutes; stir the reaction at 20-40°C for 30 minutes, pour the reaction solution into ice water, if solid precipitates, filter the solid. The solid was recrystallized with ethanol and filtered while hot. The filtrate was cooled to room temperature and filtered to obtain a yellow solid (33.60g, yield 82%). Rf=0.61 (PE:EtOAc=1:1), m.p.=88-89°C. 1H NMR (500MHz, CDCl3)δ 11.29 (s, 1H), 8.32 (s, 2H), 1.37 (s, 9H).
Apply[1]
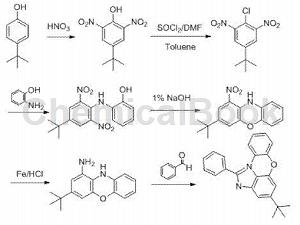
Used to prepare p-tert-butyl-o-dinitrochlorobenzene.
Dissolve 4-tert-butyl o-dinitrophenol (5g, 21mmol) in 200mL dry toluene, add DMF (2.9g) and thionyl chloride (7.4g, 62mmol) to the mixed solution, and then react The liquid was refluxed for 8 hours and then cooled. The toluene was directly removed by rotary evaporation, and the remaining liquid was poured into water. After filtration, the solid was rinsed with water and petroleum ether until the filtrate was nearly colorless to obtain a brown solid (4.58g, yield 86%). m.p.=104.2-104.6°C, Rf=0.53 (PE:EtOAc=10:1). 1H NMR (500MHz, CDCl3)δ 8.33 (s, 2H), 1.38 (s, 9H).
CN201711121670.9 uses the simple and cheap raw material p-tert-butylphenol to synthesize 4-tert-butyl-2,6-dinitrophenol, and then through a series of mild conditions, 4-(tert-butyl) is synthesized -1-Phenyl-imidazo[4,5,1-kl]phenoxazine. The invention provides a simple method for synthesizing 4-(tert-butyl)-1-phenyl-imidazo[4,5,1-kl]phenoxazine. The synthesized compound realizes the synthesis of phenoxazine and benzaldehyde. Parallel rings are expected to play important applications in the fields of organic optoelectronic materials and medicine.
Main reference materials
[1] CN201711121670.9 A preparation method of 4-(tert-butyl)-1-phenyl-imidazo[4,5,1-kl]phenoxazine

 微信扫一扫打赏
微信扫一扫打赏

