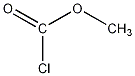
Structural formula
| Business number | 01PJ |
|---|---|
| Molecular formula | C2H3ClO2 |
| Molecular weight | 94 |
| label |
Methyl chlorocarbonate, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:79-22-1
MDL number:MFCD00000639
EINECS number:201-187-3
RTECS number:FG3675000
BRN number:605437
PubChem number:24853879
Physical property data
1. Properties: colorless and transparent liquid with a strong pungent odor. [1]
2. Melting point (℃): -61[2]
3. Boiling point (℃): 70~72[3]
4. Relative density (water = 1): 1.22[4]
5. Relative vapor density (air=1): 3.26[5]
6. Saturated vapor pressure (kPa): 14 (20℃)[6]
7. Heat of combustion (KJ/mol): -689[7]
8. Critical temperature (℃): 251.9[ 8]
9. Critical pressure (MPa): 5.36[9]
10. Octanol/water partition coefficient: 0.14[10]
11. Flash point (℃): 17.8 (CC); 24.4 (OC) [11]
12. Ignition temperature (℃): 504[12]
13. Explosion upper limit (%): 23.3[13]
14. Lower explosion limit (%): 6.7[14]
15. Solubility: insoluble in water, soluble in benzene, methanol, ether, ethanol and other organic matter Solvent. [15]
Toxicological data
1. Acute toxicity[16]
LD50: 60mg/kg (rat oral); 7120mg/kg (rabbit transdermal)
LC50: 88ppm (rat inhalation, 1h)
2. Irritation No information available
3. Subacute and chronic toxicity[17] The maximum no-effect concentration in the chronic inhalation test is 0.185 mg/m in mice3, rat is 0.197mg/m3
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 74 days (theoretical).
At 19.6°C, the hydrolysis half-life is 35 minutes.
Molecular structure data
1. Molar refractive index: 17.93
2. Molar volume (cm3/mol): 76.4
3. Isotonic specific volume (90.2K ): 174.8
4. Surface tension (dyne/cm): 27.4
5. Polarizability (10-24cm3):7.11
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity:42.9
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has tear-inducing effect. Highly toxic, flammable and corrosive. Slightly soluble in water and gradually decomposed by water. It has a tear-inducing effect, and the vapor strongly irritates the eyes and respiratory organs. It can also be absorbed through the skin and cause poisoning. The maximum allowable concentration in the workplace is 0.005mg/L.
2. This product is highly toxic, with oral LD50 in rats <50mg/kg. It has a strong irritating effect on the respiratory tract and eye mucosa. When its concentration reaches 52.8 mg/m3, it can cause tearing. The maximum allowable concentration in the air is 5mg/m3. The operating area should be well ventilated and leakage should be strictly prevented. Operators should wear protective equipment.
3. Stability[19] Stable
4. Incompatible substances[20] Acids, strong bases, alcohols, amines, water
5. Conditions to avoid contact[21] Humid air
6. Polymerization hazard[22] No polymerization
7. Decomposition products [23] Hydrogen chloride, phosgene
Storage method
Storage Precautions[24] Store in a cool, dry and well-ventilated special warehouse, and implement “two people to receive and receive, and two people to keep” “system. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from acids, alkalis, alcohols, amines, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Industrially obtained from the esterification of methanol and phosgene. When producing by the intermittent method, phosgene is introduced into methanol, and the reaction is stirred in a glass-lined reaction pot. This process has low production capacity, high raw material consumption, and poor product quality. Continuous processes are used for mass production. The continuous process generally uses a glass-lined reaction tower. Methanol is first added to the tower to control the reaction temperature to 20-30°C, and then methanol and phosgene are vented in the same direction from the bottom of the tower. The content of methyl chloroformate obtained is 93%. The yield is 94%. The reaction tail gas of this method is frozen and captured by a condenser. The phosgenation process releases a lot of heat, and the reaction tower generally uses jacket cooling to control the reaction at around 25°C. The production of each ton of methyl chloroformate consumes about 400kg of methanol and about 1500kg of phosgene.
![]()
2. [Note] Phosgene poisonous. The reaction should be carried out in a fume hood. Put anhydrous methanol in a slender glass and cool to 5°C. Slowly pass the phosgene dried with concentrated sulfuric acid into methanol. The reaction is complete when phosgene is no longer absorbed (about 1 hour). Transfer the reaction mixture to a separatory funnel and wash several times with ice water. The lower layer is separated and distilled.
![]()
Purpose
1. Pesticide raw materials, used to prepare the herbicide benzofen, fungicide carbendazim, etc.
2. Used in organic synthesis and the manufacture of pesticides, and also in the preparation of tear gas. [25]

 微信扫一扫打赏
微信扫一扫打赏

