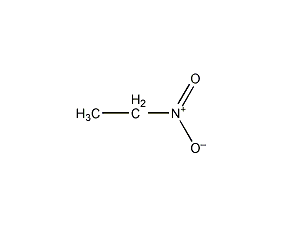
Structural formula
| Business number | 01PK |
|---|---|
| Molecular formula | C2H5NO2 |
| Molecular weight | 75.07 |
| label |
Nitroethane, Nitric oxide, Rubber and plastic additives, rocket fuel, Nitrogen-containing compound solvent |
Numbering system
CAS number:79-24-3
MDL number:MFCD00007404
EINECS number:201-188-9
RTECS number:KI5600000
BRN number:1209324
PubChem number:24853659
Physical property data
1. Properties: colorless oily liquid with unpleasant odor. [1]
2. pH value: 6.0 (0.01mol/L aqueous solution) [2]
3. Melting point (℃): -90[3]
4. Boiling point (℃): 114.0[4]
5. Relative density (water=1): 1.05[5]
6. Relative vapor density (air=1): 2.58[6]
7. Saturated vapor pressure (kPa): 2.08 (20℃)[7]
8. Heat of combustion (kJ/mol): -1362[8]
9. Critical temperature (℃): 388[9]
10. Critical pressure (MPa): 4.9 [10]
11. Octanol/water partition coefficient: 0.18[11]
12. Flash point (℃ ): 28 (CC) [12]
13. Ignition temperature (℃): 414.5[13]
14. Explosion upper limit (%): 17.3[14]
15. Explosion lower limit (%): 3.4[15]
16. Solubility: insoluble in cold water, slightly soluble in hot water, miscible in methanol, ethanol, chloroform, and ether. [16]
Toxicological data
1. Acute toxicity[17] LD50: 1100mg/kg (oral in rats); 860mg/kg (oral in mice)
2. Irritation No information available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 17.33
2. Molar volume (cm3/mol): 74.3
3. Isotonic specific volume (90.2K ): 170.2
4. Surface tension (dyne/cm): 27.5
5. Polarizability (10-24cm3): 6.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 37.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. CovalentNumber of key units: 1
Properties and stability
1. Use litmus paper to test whether the nitroethane aqueous solution is acidic. The pH of 0.01mol/L aqueous solution is 5.20; the pH of saturated aqueous solution is 3.85; the pH of water-saturated nitroethane is 3.75.
2. Like nitromethane, addition reaction occurs with carbonyl compounds in alkaline solution to generate β-nitroalcohol.
3. Form an explosive mixture with air, with an explosion limit of 3%-5% (volume). It has a high heat of adsorption, and a large amount of anhydrous nitroethane or its vapor adsorbed on activated carbon will cause combustion.
4. Stability[18] Stable
5. Incompatible substances[19] Strong reducing agents, strong acids, strong bases, amines
6. Conditions to avoid contact [20] Vibration , heating
7. Polymerization hazard[21] No polymerization
8. Decomposition products[ 22] Nitrogen oxides
Storage method
Storage Precautions[23] Store in a cool, dry and well-ventilated non-combustible warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
In industry, the direct gas phase nitrification method of low carbon alkanes is mainly used for production. Using methane as raw material, only nitromethane can be produced; using ethane as raw material, nitromethane and nitroethane can be produced; using propane as raw material, methane and nitroethane can be produced and four products: 1-nitropropane and 2-nitropropane. If ethane and propane are used as raw materials, the product ratio can be changed by changing conditions. 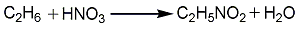
Purpose
1. This product has good dissolving ability for nitrocellulose, cellulose acetate, polyvinyl acetate, etc., and is used as a solvent and rocket for nitrocellulose, cellulose acetate, resin, wax, fat, and dyes. Dyes, also used in organic synthesis.
2. It is an excellent polar solvent with good dissolving ability for nitrocellulose, cellulose acetate, polyvinyl acetate, etc. It is used as a solvent and rocket fuel for resins, nitrocellulose, cellulose acetate, waxes, fats and dyes, etc. It is also an important intermediate for synthetic pesticides, medicines and tree dyes.
3. Used in organic synthesis as a solvent for nitrocellulose and other resins, waxes, fats, dyes, etc. [24]

 微信扫一扫打赏
微信扫一扫打赏

