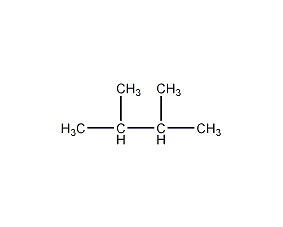
Structural formula
| Business number | 01PM |
|---|---|
| Molecular formula | C6H14 |
| Molecular weight | 86.18 |
| label |
diisopropane, Diisopropyl, gasoline additives |
Numbering system
CAS number:79-29-8
MDL number:MFCD00008925
EINECS number:201-193-6
RTECS number:EJ9350000
BRN number:1730737
PubChem number:24864705
Physical property data
1. Properties: colorless, transparent and volatile liquid
2. Density (g/mL, 25/4℃): 0.65
3. Relative vapor density (g/ mL, air = 1): 3.0
4. Melting point (ºC): -128.4
5. Boiling point (ºC, normal pressure): 58.0
6 .Refractive index (n20D) : 1.375
7. Flash point (ºC): below -29
8 .Vapor pressure (kPa, 25ºC): 31.28
9. Vapor pressure (kPa, 0ºC): 10.24
10. Explosion limit (%, V/V): 7.0
p>
11. Lower explosion limit (%, V/V): 1.2
12. Solubility: insoluble in water, soluble in alcohol, ether, chloroform, miscible in benzene and acetone.
13. Ignition point (ºC): 420
14. Heat of evaporation (kJ/mol): 27.294
15. Heat of fusion (kJ/mol): 0.812
16. Aniline point (ºC): 71.9
17. Heat of formation (25 ºC, liquid) / (kJ·mol): -207.16
18. Specific heat capacity (27ºC, constant pressure)/[kJ/(kg·K)]: 2.00
19. Critical temperature (K): 226.85
20. Critical pressure (MPa ): 3.15
21. Critical density (g·cm-3): 0.239
22. Critical volume (cm3·mol-1): 361
23. Critical compression factor: 0.279
24. Eccentricity factor: 0.248
25 .Lennard-Jones parameter (A): 9.085
26. Lennard-Jones parameter (K): 232.9
27. Solubility parameter (J·cm-3)0.5: 14.353
28. van der Waals area (cm2·mol-1): 9.620×109
29. van der Waals volume (cm3·mol-1): 68.240 p>
30. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4183.58
31. Gas phase standard claimed heat (enthalpy) (kJ· mol-1): -178.28
32. Gas phase standard entropy (J·mol-1·K-1 ): 365.93
33. Gas phase standard formation free energy (kJ·mol-1): -4.4
34. Gas phase standard hot melt (J· mol-1·K-1): 139.41
35. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 ): -4154.46
36. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -207.40
37 .Liquid phase standard entropy (J·mol-1·K-1): 278.85
38. Liquid phase standard free energy of formation (kJ· mol-1): -7.76
39. Liquid phase standard hot melt (J·mol-1·K-1):189.04
Toxicological data
Other multiple dose toxicity data: Rat caliber TCLO: 10mg/kg/4W-I
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Mount�Refractive index: 29.76
2. Molar volume (cm3/mol): 128.3
3. Isotonic specific volume (90.2K): 265.6
4. Surface tension (dyne/cm): 18.3
5. Dielectric constant (F/m): 1.88
6. Polarizability (10-24cm3): 11.79
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 21
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The chemical properties are relatively stable, and halogenation reaction occurs under the action of sunlight or ultraviolet light to generate halogen derivatives. During the nitration reaction, nitro compounds are produced. Reacts strongly with oxidants and may cause combustion and explosion when exposed to open flames or high heat.
2. Exist in smoke.
Storage method
This product should be kept sealed and dry.
Synthesis method
It is produced by the reaction of ethylene and isobutane. Purification method: Unsaturated compounds are removed by washing with concentrated sulfuric acid. Moisture can be removed with calcium chloride, phosphorus pentoxide, metallic sodium or solid desiccant.
Purpose
As an additive to aviation gasoline and motor gasoline, it is also used in organic synthesis and as a gas chromatography comparison sample.

 微信扫一扫打赏
微信扫一扫打赏

