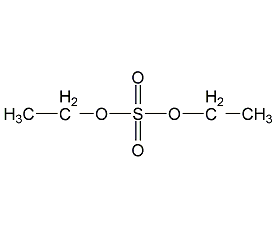
Structural formula
| Business number | 01DD |
|---|---|
| Molecular formula | C4H10O4S |
| Molecular weight | 154 |
| label |
None yet |
Numbering system
CAS number:64-67-5
MDL number:MFCD00009099
EINECS number:200-589-6
RTECS number:WS7875000
BRN number:1209714
PubChem number:24893316
Physical property data
1. Properties: colorless oily liquid with a slight ether smell. [1]
2. Melting point (℃): -24.0[2]
3. Boiling point (decomposition, ℃ ): 208[3]
4. Relative density (water = 1): 1.18[4]
5. Relative vapor density (air=1): 5.31[5]
6. Saturated vapor pressure (kPa): 0.13 (47.0℃)[6]
7. Critical pressure (MPa): 6.48[7]
8. Octanol/water partition coefficient: 1.14[8]
9. Flash point (℃): 78.33; 104 (CC) [9]
10. Ignition temperature: 436[10]
11. Explosion upper limit (%): No data yet
12. Explosion lower limit (%): 4.1[11]
13. Solubility: Insoluble in water, soluble in ethanol and ether. [12]
Toxicological data
1. Acute toxicity[13] LD50: 880mg/kg (rat oral); 600mg/kg (rabbit dermal )
2. Irritation[14]
Rabbit transdermal: 10mg ( 24h), severe stimulation (open stimulation test).
Rabbit eye: 2 mg, severe irritation.
3. Mutagenicity[15] Microbial mutagenicity: Salmonella typhimurium 5mg/dish; Escherichia coli 10mmol/L. DNA damage: Human HeLa cells 20mg. Cytogenetic analysis: human lymphocytes 300 μmol/L. Unprogrammed DNA synthesis: rat liver 100 μmol/L.
4. Carcinogenicity[16] IARC Carcinogenicity Comment: G2A, possible human carcinogen.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[17]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3. Non-biodegradability[18]
Photooxidation half-life in air (h): 3.6 ~36
First-order hydrolysis half-life (h): 1.7
Molecular structure data
1. Molar refractive index: 32.85
2. Molar volume (cm3/mol): 128.3
3. Isotonic specific volume (90.2K ): 313.8
4. Surface tension (dyne/cm): 35.7
5. Polarizability (10-24cm3): 13.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecular extremes��Surface area 61
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 130
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. If exposed to high temperatures, it may decompose violently, causing container rupture or explosion. Reacts violently with potassium tert-butoxide. It easily decomposes when exposed to moisture and produces corrosive liquid sulfuric acid.
2. Stability[19] Stable
3. Incompatible substances[20] Strong oxidant, strong alkali, water
4. Conditions to avoid contact[21] Humid air, heat
5. Polymerization hazard[22] No polymerization
6. Decomposition products[23] Sulfur oxide
Storage method
Storage Precautions[24] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are many production methods. 1. Obtained from the reaction of ethanol and sulfuric acid. 2. By the action of ether and chlorosulfonic acid. 3. Heat monoethyl sulfate or distill it in the presence of a dehydrating agent to obtain diethyl sulfate. 4. Diethyl sulfate is produced by reacting ethylene with sulfuric acid.
Purpose
1. Diethyl sulfate is a highly reactive ethylation agent and is widely used in the production of dyes, medicines, pesticides and other fine chemical products. It is also used in the synthesis of quaternary ammonium salts, as dehydrating agent, volatile oil extraction agent, etc.
2. Used as ethylating agent in organic synthesis. [25]

 微信扫一扫打赏
微信扫一扫打赏

