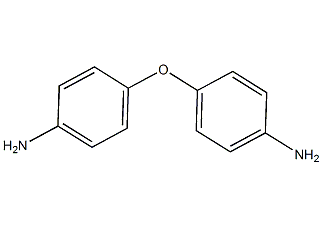
Structural formula
| Business number | 02L3 |
|---|---|
| Molecular formula | C12H12N2O |
| Molecular weight | 200.24 |
| label |
para-amino diphenyl ether, 4,4′-diaminophenyl oxide, 4,4′-bis(phenylamino)ether, Oxylene diphenylamine, 4-aminophenyl ether, 4,4′-bis(aminophenyl)ether bis(4-aminophenyl)ether, 4,4′-oxybisbenzenamine, Hardener, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:101-80-4
MDL number:MFCD00007863
EINECS number:202-977-0
RTECS number:BY7900000
BRN number:475735
PubChem number:24854926
Physical property data
1. Properties: Colorless crystal or white powder, odorless.
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 186~187
5. Boiling point (ºC, normal pressure): >300 p>
6. Boiling point (ºC, 0.1mmHg): 190
7. Refractive index: Undetermined
8. Flash point (ºC): 218
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 240ºC ): 10
12. Saturated vapor pressure (kPa, 25ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Insoluble in water, easily soluble in hydrochloric acid, insoluble in benzene.
Toxicological data
Acute toxicity: Rat oral LD50: 725mg/kg
Toxic, inhalation of vapor or powder or absorption through skin can cause poisoning.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 61.16
2. Molar volume (cm3/mol): 164.5
3. Isotonic specific volume (90.2K ): 450.0
4. Surface tension (dyne/cm): 55.9
5. Dielectric constant:
6. Dipole moment (10-24cm3) :
7. Polarizability: 24.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 61.3
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 162
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents. Toxic, production equipment should be sealed, and good ventilation should be maintained at the production site to prevent dust from flying. Operators must wear protective equipment
Poisonous. Can cause cancer. Poisoning can occur if the vapor or powder is inhaled or absorbed through the skin. It can damage the human nervous system, cause blood to form denatured hemoglobin, and have hemolysis. Production equipment should be airtight. The production site should be well ventilated to prevent dust. Operators should wear protective equipment.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should also be available to contain spills. Packed in iron drums lined with plastic bags, 20kg per drum. Store in a cool, dry place. Fireproof, moistureproof and sunproof. Storage and transportation according to regulations for flammable and toxic substances
Synthesis method
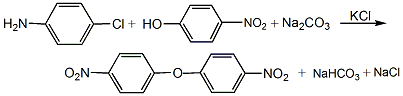
(1) Condensation reaction Add 515kg of solvent nitrobenzene into the stirred reactor, then add 145kg of nitrochlorobenzene, 123.5kg of p-nitrophenol, 95.7kg of sodium carbonate and 6.18kg of potassium chloride in sequence while stirring. In the reaction kettle, the temperature was raised to 210-215°C with stirring, and then the reaction was stirred and maintained at this temperature for 24 hours. After the condensation reaction is completed, cool it, pump the condensate to the distillation device, and perform vacuum distillation first. When 350 to 400L of nitrobenzene has evaporated, change to steam distillation until there is no nitrobenzene in the distillate. The residue is filtered with suction, and the filtrate is washed with water until colorless to obtain 4,4’-dinitrodiphenyl ether.
(2) Reduction reaction Add 918kg of alcohol into the reduction reaction kettle, add 10kg of ammonium chloride and 50kg of iron filings while stirring, add 25kg of 4,4′-dinitrodiphenyl ether when the temperature reaches 95°C, and stir for 0.5h. Then add the second batch of 40kg iron filings and 20kg 4,4’-dinitrodiphenyl ether, and stir for 0.5h. Then add the third batch of 40kg of iron filings and 20kg of 4,4’-dinitrodiphenyl ether, still maintaining the reaction temperature at 100°C, and take samples for 1.5 hours to analyze the reaction end point. After the reaction is completed, add 12 kg of sodium bisulfite, stir and dissolve, then use a tubular suction filter to pump the reaction material while it is hot into the crystallization kettle for cooling and crystallization for about 8 hours. Then it is filtered, the filtrate butanol is distilled and recycled, and the filter cake is washed with water until there is no butanol to obtain crude 4,4’-diaminodiphenyl ether.
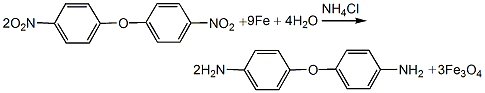
(3) Product Refining 340kg Water and 60kg of 30% hydrochloric acid are added to the dissolution tank. Under stirring, add crude 4,4′-diaminodiphenyl ether, 1.5kg of citric acid, and 1kg of sodium bisulfite. Heat to 60°C, and then add 9kg of activated carbon. Continue to raise the temperature to 80°C and maintain for 0.5h. After cooling, filter, add the filtrate to the neutralization tank, add 0.5kg of sodium bisulfite while stirring, then slowly add ammonia water, keep the temperature below 30°C, and neutralize until the pH value of the material is 8 to 9, then 4,4 ‘-diaminodiphenyl ether crystals precipitated from the solution. After filtration, the filter cake is washed with water until neutral and free of chloride ions, and then vacuum-dried after filtration.
Add the dried 4,4′-diaminodiphenyl ether into the sublimator, vacuum to below 26.66Pa, heat to 185~190℃, 4,4′-diaminodiphenyl ether The ether vaporizes and enters the crystallization chamber, where it is cooled and crystallized. The temperature of the crystallization chamber is controlled at 135 to 145°C. When the temperature of the crystallization chamber drops significantly, the sublimation is completed. The sublimator is cooled until the temperature of the crystallization chamber drops to 50°C for discharging, and the finished product is obtained. The sublimation operation takes about 10 hours per batch.
Purpose
It is used to make heat-resistant plastics such as polyimide resin, polymaleimide resin, polyamide-imide resin, polyester-imide resin, epoxy resin, polyurethane, etc., and is also used as a cross-linking agent. This product is used as a curing agent for epoxy resin. The cured product has a high thermal deformation temperature, good thermal stability, and excellent chemical resistance and water resistance.
This product is the raw material for heat-resistant plastic polyamide-imide resin, polyester-imide resin, polyimide resin, polymaleimide resin, etc. Used as a curing agent for epoxy resin, the cured product has a high thermal deformation temperature, good thermal stability, and excellent chemical resistance and water resistance. It is also the raw material for heat-resistant plastics such as polyamide imide resin, polyester imide resin, polyimide resin, and polymaleimide resin.
Raw materials for imide resin, polyimide resin, polymaleimide resin, etc.

 微信扫一扫打赏
微信扫一扫打赏

