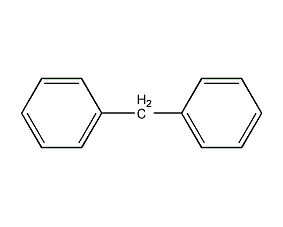
Structural formula
| Business number | 02L4 |
|---|---|
| Molecular formula | C13H12 |
| Molecular weight | 168.24 |
| label |
benzylbenzene, diphenylmethane, 1,1-diphenylmethane, Benzyl benzene, 1,1′-Methylenebis[benzene], Ditan, artificial flavors, aromatic compounds |
Numbering system
CAS number:101-81-5
MDL number:MFCD00004781
EINECS number:202-978-6
RTECS number:PA8990000
BRN number:1904982
PubChem number:24854401
Physical property data
1. Properties: colorless needle-shaped crystals with scent of geranium-sweet orange.
2. Density (g/mL, 20℃): 1.01 (liquid); 1.34 (solid)
3. Relative density (d264) : 1.001
4. Melting point (ºC): 26.5
5. Boiling point (ºC, normal pressure): 265
6. Boiling point (ºC, 0.1mmHg):
7. Refractive index (n/20D): 1.5753
8. Flash point (ºC): 130
9. Relative density (25℃, 4℃): 0.100827
10. Refractive index at room temperature (n25): 1.5752 d
11. Vapor pressure (mmHg, 77ºC): <1
12. Critical temperature (K): 486.85
13. Critical Pressure (MPa): 2.71
14. Critical density (g·cm-3): 0.299
15. Critical volume (cm3 ·mol-1): 563
16. Critical compression factor: 0.241
17. Eccentricity factor: 0.462
18. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -6920.25
19. Solubility: insoluble in water, soluble in ethanol and ether , chloroform and other organic solvents.
20. Solubility parameter (J·cm-3)0.5: 19.415
21. van der Waals area ( cm2·mol-1): 1.042×1010
22. van der Waals volume (cm3·mol-1): 93.410
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1) : -6987.74
24. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): 89.66
25. Liquid phase standard entropy ( J·mol-1·K-1): 301.7
26. Liquid phase standard free energy of formation (kJ·mol-1 ): 255.5
27. Liquid phase standard hot melt (J·mol-1·K-1): 195 p>
28. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 157.15
29. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -6902.05
30. Crystal Phase standard claims heat (enthalpy) (kJ·mol-1): 71.5
31. Crystal phase standard entropy (J·mol-1· K-1): 239.3
32. Crystal phase standard formation free energy (kJ·mol-1): 255.8
33. Crystal phase standard hot melt (J·mol-1·K-1): 233
Toxicological data
Oral administration of LDL 05000mg/kg to rats. For the rest, see biphenyl.
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. Can cause pollution to water bodies and the atmosphere.
Molecular structure data
1. Molar refractive index: 55.56
2. Molar volume (cm3/mol): 168.8
3. Isotonic specific volume (90.2K ): 418.3
4. Surface tension (dyne/cm): 37.6
5. Dielectric constant: 2.66
6. Dipole moment (10-24cm3):
7. Polarizability: 22.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 111
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2.Oral administration of LDL05000mg/kg to white rats. For the rest, see biphenyl.
3. Exists in burley tobacco leaves and oriental tobacco leaves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. Store in a cool and ventilated warehouse, away from fire and heat sources, and keep packaging intact. Should be kept sealed.
Synthesis method
1. Obtained from the reaction of benzyl chloride and benzene catalyzed by aluminum amalgam (or anhydrous aluminum trichloride, or zinc chloride). First heat the dried benzene and aluminum amalgam, then slowly add benzyl chloride, reflux for 2 hours, cool and stir with water, let stand to remove water, the remainder is washed with 5% alkali solution, washed with water, and finally distilled under reduced pressure to obtain the finished product.
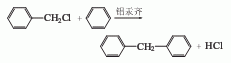
2. Tobacco: BU, 14 ;OR,26.
Purpose
Manufacture dyes and spices, and also used as solvents. A substitute for geranium oil, suitable for preparing soap essence.

 微信扫一扫打赏
微信扫一扫打赏

