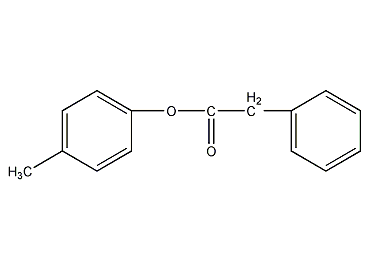
Structural formula
| Business number | 02LC |
|---|---|
| Molecular formula | C15H14O2 |
| Molecular weight | 226.27 |
| label |
p-Tolyl Phenylacetate, Phenylacetic Acid p-Cresyl Ester, p-Cresyl Phenylacetate, Phenylacetic Acid p-Tolyl Ester, artificial flavors |
Numbering system
CAS number:101-94-0
MDL number:MFCD00025983
EINECS number:202-990-1
RTECS number:CY1679750
BRN number:None
PubChem number:24901199
Physical property data
1. Characteristics: White crystals, with a fragrance like hyacinth and narcissus, and a fragrance like honey.
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 74-76
5. Boiling point (ºC, normal pressure): 310
6. Boiling point (ºC, 1.5mmHg): 148
p>
7. Refractive index: Undetermined
8. Flash point (ºC): >100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water and glycerin, slightly soluble in propylene glycol, soluble in in ethanol.
20. Freezing point (℃): 73.5
Toxicological data
- Acute toxicity: oral rat: LD50>5g/kg; skin rabbit: LD50>2g/kg
- This substance was applied to normal or damaged rabbit skin under closed conditions for 24 hours and found to have Moderately irritating. If a petroleum jelly preparation diluted to 4% is used in a closed skin contact test on humans, no irritation will occur after two days. This preparation has not produced any allergic reactions when tested to the maximum extent possible on humans.
Ecological data
None yet
Molecular structure data
Molecular property data:
1. Molar refractive index: 66.90
2. Molar volume (cm3/mol): 204.0
3.� Isotonic specific volume (90.2K): 518.5
4. Surface tension (dyne/cm): 41.7
5. Dielectric constant:
6. Even Polar distance (10-24cm3):
7. Polarizability: 26.52
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 235
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White crystal, soluble in 4 to 5 volumes of 95% ethanol, insoluble in water, propylene glycol and glycerol. It has a salty, sweaty animal aroma. The aroma is strong and rich. When extremely diluted, it has a narcissus-like aroma and a musky animal scent, which is also like lily of the valley, hyacinth, and narcissus.
Storage method
Store in a cool place away from light.
Synthesis method
1. Made from the esterification of p-cresol and phenylacetic acid.
It is completed by the condensation of sodium phenolate or freshly steamed methylphenol and phenylacetyl chloride; input ratio: phenylacetic acid: p-cresol: sulfinyl chloride = 65:52:60. First, heat the phenylacetic acid under reduced pressure to remove moisture, then add sulfenyl chloride dropwise under normal pressure and stirring to drain the generated hydrogen chloride gas, and add dropwise for about 2 hours until no hydrogen chloride gas is generated. Then add p-cresol and raise the temperature to 90°C with stirring until no hydrogen chloride gas is generated. After cooling, neutralize with (3:1) alkaline water, keep the reaction temperature below 40°C, coarse crystals will precipitate, and the unreacted raw materials will be recovered from the mother liquor. The crude crystals were washed with water and finally recrystallized with 95% ethanol to obtain the product.
2.It is prepared by the condensation of sodium phenolate or methylphenol and phenylacetyl chloride. After the reaction is completed, the crude product is washed with water and recrystallized to obtain the product.
Purpose
1.P-Cresol phenylacetate In addition to having similar aroma characteristics to p-cresol acetate, its uses are slightly better. As early as 80 years ago, it has been widely used in the preparation of various flavors, especially suitable for the preparation of fresh and delicate fragrances such as narcissus, jasmine grandiflorum, gardenia, ylang-ylang, tuberose, lily of the valley, hyacinth, etc. Also suitable as a modifier for artificial musk. Usually the concentration in the final product is soap 0.01% ~ 0.1%; detergent 0.001% ~ 0.01%; perfume 0.08% ~ 0.3%; creams and liquids 0.005% ~ 0.03%. Food flavors are used to prepare flavors such as honey, cream, nuts, caramel, etc. The dosage in the application medium is 1.66 to 0.87 mg/kg for soft drinks and iced foods and 4.8 to 5.4 mg/kg for candies and baked goods.
2.Used for the preparation of various flavors, especially suitablefor narcissus and gardenia. The preparation of fragrances such as jasmine, ylang-ylang, tuberose, lily of the valley, hyacinth, etc. Also suitable as a modifier for artificial musks. Food flavors are used to prepare flavors such as honey-sweet cream, nuts, caramels, etc. The dosage for soft drinks and iced foods is 1.66~0.87mg/kg; for candies and baked goods, the dosage is 4.8~5.4mg/kg. In daily products, it is usually used as soap flavor, detergent flavor, perfume flavor, cream flavor, etc.

 微信扫一扫打赏
微信扫一扫打赏

