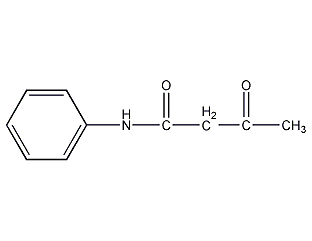
Structural formula
| Business number | 02LH |
|---|---|
| Molecular formula | C10H11NO2 |
| Molecular weight | 177.20 |
| label |
Acetoacetanilide, AAA, Acetoacetic anilide, Acetoacetylaniline, a-Acetylacetanilide, 3-Oxo-N-phenylbutanamide, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:102-01-2
MDL number:MFCD00008780
EINECS number:202-996-4
RTECS number:AK4200000
BRN number:473419
PubChem number:24878321
Physical property data
1. Appearance: White crystalline solid
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, Air=1): Undetermined
4. Melting point (ºC): 86
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): 163
9. Ratio Optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
p>
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble Slightly soluble in water, in ethanol, chloroform, ether, hot benzene, hot petroleum ether, acid and alkali hydroxide solutions. It turns purple when encountering ferric chloride.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2450mg/kg; Rat peritoneal cavity LD50: 800mg/kg; Rat subcutaneous LD50: 7mg/kg; Mouse oral LD50: 3400mg/kg; Mouse peritoneal cavity LD50: 300mg/kg; Oral LDLo for cats: 500mg/kg; &nbSP; rabbit passing LD50: 3925mg/kg; guinea pig skin contact LD50:> 1mg/kg; 2. Other multiple dose toxicity: Rat via the mouth TDLO: 14024mg/kg/14d-C
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 49.82
2. Molar volume (cm3/mol): 152.6
3. Isotonic specific volume (90.2K ): 395.6
4. Surface tension (dyne/cm): 45.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 8
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 195
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2. When using, avoid inhaling the dust of this product and avoid contact with eyes and skin.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. 2.Packed in plastic bags or plastic-coated fiberglass bags, 30kg per bag. Store airtight in a cool, ventilated, dry place, handle with care when handling. Store and transport according to general chemical regulations.
Synthesis method
Obtained from the reaction between diketene and aniline: react aniline and diketene at a temperature of 0-15°C to form acetoacetanilide, which is then filtered and dried to obtain the finished product. Compared with the acylation using ethyl acetate, the acylation of diketene has the advantages of simple process, high yield and good quality. Raw material consumption quota: aniline (99.5) 548kg/t, diketene (96%) 501kg/t. Laboratory preparation can be carried out as follows: put 46g (0.5 mol) anhydrous aniline and 125ml anhydrous benzene in a 500ml three-necked flask. Add diketene’s benzene solution (42g, 0.5mol diketene plus 75ml benzene) dropwise under stirring, and finish dropping within half an hour. Reflux the reactant for 1 hour, and then evaporate the benzene. The residue is dissolved in hot ethanol aqueous solution (50% ethanol aqueous solution 500ml). Acetoacetanilide precipitates on cooling and is filtered. The filtrate was diluted with 250 ml, cooled, and filtered to obtain part of the product. A total of 65 g was obtained, with a yield of 75% and a melting point of 82-83.5°C. The crude product was recrystallized with 300 ml of 50% ethanol to obtain 55 g of pure product with a melting point of 84-85°C.
The preparation method is to start stirring in the reaction kettle, lower the temperature by 0 to 15°C, add aniline and diketene through a flow meter at a certain flow ratio at the same time, complete the addition in about 2 hours, and then add the mixture at 10 to 15°C. Stir for 2 h, filter and dry with suction to obtain the finished product.
![]()
Purpose
Dye intermediates, used to manufacture dyes such as pyrazolone, bright yellow 5G, acidic complex yellow GR, neutral dark yellow GL, neutral orange RL, sweat sand yellow G, pigment light-fast yellow G, etc. Also used in the manufacture of medicines.

 微信扫一扫打赏
微信扫一扫打赏

