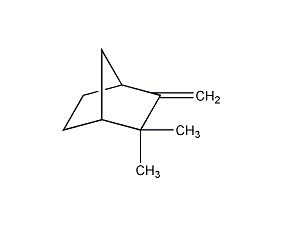
Structural formula
| Business number | 01Q8 |
|---|---|
| Molecular formula | C10H16 |
| Molecular weight | 136.23 |
| label |
2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane, 2,2-dimethyl-3-methyleneorbornane, Preparation of mint and spice flavors |
Numbering system
CAS number:79-92-5
MDL number:MFCD00066603
EINECS number:201-234-8
RTECS number:EX1055000
BRN number:None
PubChem number:24869106
Physical property data
1. Characteristics: colorless or yellowish crystals with the smell of camphor. [1]
2. Melting point (℃): 51~52[2]
3. Boiling point (℃) :158.5~159.5[3]
4. Relative density (water=1): 0.84[4]
5 .Saturated vapor pressure (kPa): 5.32 (75.7℃)[5]
6. Heat of combustion (KJ/mol): -6139.6[6]
7. Critical pressure (MPa): 2.75[7]
8. Octanol/water partition coefficient: 4.02[8 ]
9. Flash point (℃): 33 (CC); 42 (OC) [9]
10. Solubility : Insoluble in water, slightly soluble in ethanol, soluble in ether, miscible in fixed oil. [10]
Toxicological data
1. Acute toxicity[11]
LD50: >5g/kg (rat oral) ;>2500mg/kg (rabbit transdermal)
LC50: 17100mg/m3 (rat inhalation, 4h)
2. Stimulation Sex No information yet
Ecological data
1. Ecotoxicity[12]
LC50: 1.9mg/L (96h) (red perch ); 46mg/L (24h) (water flea); 2.03mg/L (48h) (medaka)
2. Biodegradability No data yet
3. Non-biodegradability[13] In the air, when the concentration of hydroxyl radicals is 5.00×105pcs/cm3, the degradation half-life is 7.2h (theoretical).
4. Bioconcentration[14] BCF: 432~922 ( Carp, exposure concentration 15mg/L, exposure time 8 weeks); 606~1290 (carp, exposure concentration 1.5mg/L, exposure time 8 weeks)
Molecular structure data
1. Molar refractive index: 43.76
2. Molar volume (cm3/mol): 153.0
3. Isotonic specific volume (90.2K ): 349.0
4. Surface tension (dyne/cm): 27.0
5. Dielectric constant (F/m): 2.49
6. Polar Chemical rate (10-24cm3): 17.34
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 177
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 2
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible materials[16] Strong oxidants, acids, halogenated hydrocarbons, halogens, etc.
3. Hazards of aggregation[17] No aggregation
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 35℃. The packaging is sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is produced by separating nutmeg oil or nutmeg oil (content up to 80%).
Purpose
1. Synthetic camphor and borneol substitutes. Can be used as an insecticide.
2. Mainly used for preparing mint and spice flavors.
3. Used in the pharmaceutical industry as a preservative and a raw material for the synthesis of camphor, spices, etc. [19]

 微信扫一扫打赏
微信扫一扫打赏

