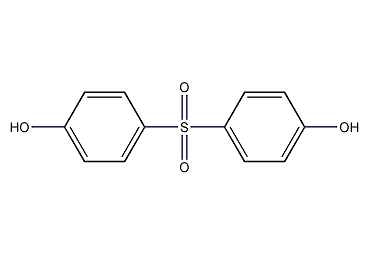
Structural formula
| Business number | 01QK |
|---|---|
| Molecular formula | C12H10O4S |
| Molecular weight | 250.27 |
| label |
4-Hydroxyphenyl sulfone, Bisphenol S, 4,4′-Sulfonyldiphenol, Fixative |
Numbering system
CAS number:80-09-1
MDL number:MFCD00002350
EINECS number:201-250-5
RTECS number:SM8925000
BRN number:2052954
PubChem number:24846593
Physical property data
1. Properties: White needle-like crystals.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 240-241℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa) : Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12 . Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
p>
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Easily soluble in aliphatic hydrocarbons, soluble in Slightly soluble in alcohol and ether, slightly soluble in aromatic hydrocarbons, hardly soluble in water.
Toxicological data
Rat caliber LD50: 4556mg/kg; rat abdominal cavity LD50: 400mg/kg;
Mouse caliber LD50: 1600mg/kg; mouse abdominal cavity LD50: 200mg/kg;
Rabbit caliber LD50: 4700mg/kg; Rabbit skin LD50: >10250mg/kg;
Pig skin LD50: >1mg/kg;
2. Other multi-dose toxicity data
Rat caliber TDL0: 15030mg/kg/13D-I;
3. Neurotoxicity
Rabbit skin test: 500mg/24H; Rabbit eye test: 100mg/24H
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 63.33
2. Molar volume (cm3/mol): 174.6
3. Isotonic specific volume (90.2K ): 487.5
4. Surface tension (dyne/cm): 60.7
5. Polarizability (10-24cm3): 25.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecular polar surface area (TPSA): 74.6
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 302
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
The molecule of this product contains two hydroxyl groups and a sulfone group with strong electron withdrawal, so it is more acidic than other phenols. It has heat resistance, oxidation resistance and light stability.
Storage method
1. This product should be sealed and stored away from light.
2. Toxic or corrosive substances such as phenol and sulfuric acid are used in production. Production equipment must be sealed and operators should wear labor protection supplies.
Synthesis method
Bisphenol S is obtained from the reaction of phenol and sulfuric acid. 4,4′-dihydroxydiphenyl sulfide can also be prepared by reacting phenol with sulfur dichloride, and then oxidized with hydrogen peroxide to obtain bisphenol S.
Purpose
4,4′-Dihydroxydiphenylsulfone is mainly used for color fixation agent. In addition, it can be used as a plating bath additive, leather tanning agent, dispersant for high-temperature dyeing of disperse dyes, phenolic resin hardening accelerator, resin flame retardant, etc. It is also an intermediate for pesticides, dyes and auxiliaries. As a substitute for bisphenolA, it can be used as a raw material for polycarbonate, epoxy resin, polyester, phenolic resin, as well as polysulfone and polyethersulfone. This product is also used in the manufacture of color photographic materials, photographic contrast enhancers, heat-sensitive recording materials (developing agents), daily surfactants and efficient deodorants, etc. The color-fixing agent A can be prepared from bisphenol S (foreign trade name Cibatex PA).

 微信扫一扫打赏
微信扫一扫打赏

