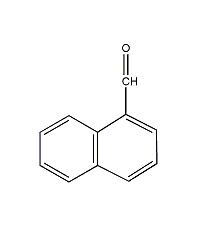
Structural formula
| Business number | 01E9 |
|---|---|
| Molecular formula | C11H8O |
| Molecular weight | 156.18 |
| label |
α-naphthaldehyde, α-Naphthaldehyde |
Numbering system
CAS number:66-77-3
MDL number:MFCD00004003
EINECS number:200-633-4
RTECS number:QJ0190000
BRN number:386082
PubChem number:24897459
Physical property data
1. Character: light yellow crystal
2. Density (g/mL, 25/4℃): 1.15
3. Relative vapor density (g/mL, air =1):>1
4. Melting point (ºC): 1-2
5. Boiling point (ºC, normal pressure): Uncertain
6 . Boiling point (ºC, 15mmHg): 160-161
7. Refractive index: 1.652
8. Flash point (ºF): >230
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature ( ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Soluble in ethanol, ether, acetone, insoluble in water.
Toxicological data
Acute toxicity: rat oral LD50: 667 mg/kg; mouse unknown route LD50: 1100 mg/kg; guinea pig Administration onto the skin LD50: >20 mL/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 50.84
2. Molar volume (cm3/mol): 135.2
3. Isotonic specific volume (90.2K ): 356.1
4. Surface tension (dyne/cm): 48.1
5. Polarizability (10-24cm3):20.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 164
10. Number of isotope atoms: 0
11. Determine the atomic stereocenterQuantity: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain chemical bonds Number of stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored away from light.
Synthesis method
1. Preparation method:
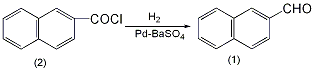
In a reaction flask equipped with a stirrer, dropping funnel, ventilation tube (extending into the bottom of the reaction flask), and a reflux condenser, add 57g (0.3mol) of β-naphthoyl chloride (2) and 200mL of metallic sodium-dried dichloride. Toluene, 6gPd-BaSO4 catalyst. Replace the air in the reaction bottle with hydrogen. Pour hydrogen gas into the oil bath at 140-150°C with vigorous stirring at a ventilation rate of 1-2 bubbles/second. Hydrogen chloride gas escapes (can be absorbed by alkali). The reaction takes about 2 hours to complete (can be judged by checking whether hydrogen chloride escapes). Cool and filter off the catalyst. Activated carbon decolorizes. The xylene is distilled off. Then distill under reduced pressure, collect the fraction at 147~149℃/2.0kPa, and solidify after cooling to obtain 19g of β-naphthaldehyde (1), mp59~60℃. Yield 80%. [1]
2. Preparation method:
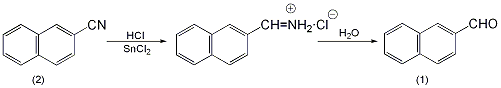
Into a reaction bottle equipped with a stirrer, dropping funnel, ventilation tube, and reflux condenser, add 400 mL of anhydrous ether and 76 g of anhydrous stannous chloride. Dry hydrogen chloride gas was introduced under cooling until saturated, and the reaction liquid was divided into two layers. A solution of 30.6g (0.2mol) of β-naphthylcarbonitrile (2) dissolved in 200mL of anhydrous ether was added dropwise under stirring, and then dry hydrogen chloride gas was introduced. Stir quickly and let sit overnight. The solid was filtered off and washed with diethyl ether. Add the solid matter to another reaction bottle, add water, and perform steam distillation. The distillate cools and a solid precipitates. After suction filtration and drying, 25g of β-naphthaldehyde (1) was obtained, with a yield of 80%. Distill under reduced pressure, collect the fraction at 156~158℃/2.0kPa, and solidify after cooling. mp57~58℃[2]
Purpose
Used in organic synthesis. Resins and medicines.

 微信扫一扫打赏
微信扫一扫打赏

