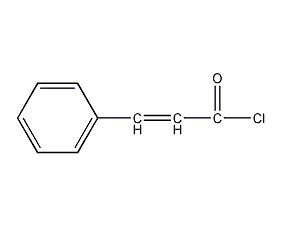
Structural formula
| Business number | 02MQ |
|---|---|
| Molecular formula | C9H7ClO |
| Molecular weight | 166.6 |
| label |
trans-3-phenylacryloyl chloride, trans-3-Phenylacryloyl chloride |
Numbering system
CAS number:102-92-1
MDL number:MFCD00000732
EINECS number:203-065-5
RTECS number:None
BRN number:606265
PubChem number:24893025
Physical property data
1. Properties: White or yellowish needle-like crystals.
2. Density (g/mL, 25℃): 1.167
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 35-37
5. Boiling point (ºC, normal pressure): 256-258
6. Boiling point (ºC, mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in petroleum ether, carbon tetrachloride and heat Ethanol, insoluble in water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 47.02
2. Molar volume (cm3/mol): 138.9
3. Isotonic specific volume (90.2K ): 353.7
4. Surface tension (dyne/cm): 42.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 157
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units:1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Obtained from the reaction of sodium cinnamate and oxalyl chloride. Make a solution of oxalyl chloride and benzene, and add the dried sodium cinnamate in multiple portions with occasional stirring. Add the next addition after each addition, allowing the reaction to release carbon dioxide. Then reflux for 2h. After cooling slightly, the generated sodium chloride and trace amounts of unreacted sodium cinnamate are filtered out. The filtrate is first evaporated to remove benzene under normal pressure, and then distilled under reduced pressure to collect the 131°C (1.47kPa) fraction to obtain the product with a yield of 75-90%. When purification is required, carbon tetrachloride can be used to recrystallize it. Cinnamic acid reacts with thionyl chloride to produce cinnamoyl chloride.
Purpose
Used as an intermediate in organic synthesis; a reagent for measuring trace amounts of moisture.

 微信扫一扫打赏
微信扫一扫打赏

