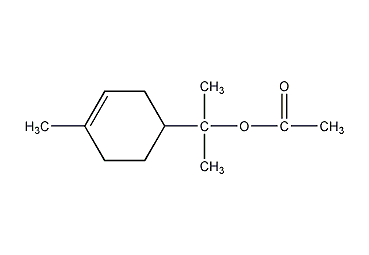
Structural formula
| Business number | 01QT |
|---|---|
| Molecular formula | C12H20O2 |
| Molecular weight | 196.29 |
| label |
1-Terpene-8-ol acetate, Terpinyl acetate, a-Terpine acetate, α-Terpineyl acetate, 1-p-Menthen-8-yl acetate, Terpineol acetate, Flavor raw materials |
Numbering system
CAS number:80-26-2
MDL number:MFCD00037155
EINECS number:201-265-7
RTECS number:OT0200000
BRN number:3198769
PubChem number:24888689
Physical property data
1. Properties: colorless oily liquid with lemon and lavender aroma.
2. Density (g/mL, 25/4℃): 0.962
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): -50 ℃
5. Boiling point (ºC, normal pressure): 220, 87~88ºC (0.4kpa)
6. Boiling point ( ºC, 5.2kPa): Uncertain
7. Refractive index (n21D): 1.4689
8. Flash point (ºC): 100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) distribution coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Soluble in organic solvents such as ethanol, ethyl acetate, diethyl ether, cyclohexane, etc., but insoluble in water.
Toxicological data
1. Acute toxicity
Rat caliber LD50: 5075mg/kg;
Mouse caliber LC50: 4800mg/kg;
Mammal inhalation LC50 :>1mg/m3
2. Other multiple dose toxicity data
Rat inhalation TCL0: 130 mg/m3/4H/17W-I
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 56.58
2. Molar volume (cm3/mol): 203.9
3. Isotonic specific volume (90.2K ): 484.7
4. Surface tension (dyne/cm): 31.8
5. Polarizability (10-24cm3): 22.43
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 251
10. IsotopesNumber of atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in essential oils such as lavender, cardamom, eucalyptus, and bitter orange, usually acetic acid A mixture of α-terpineol and early terpineol acetate, mainly α-terpineol acetate.
2. Exist in flue-cured tobacco leaves.
Storage method
Packaging in galvanized iron drums. Store in a cool and dry place. Keep away from fire and heat sources. Do not store together with flammable and explosive materials.
Synthesis method
1. It is mainly produced using turpentine as raw material.
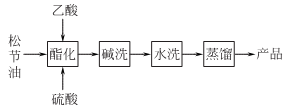
 2.Add the mixed aqueous solution of acetic acid and sulfuric acid into turpentine, place it at 30-40°C for several hours, and then allow the oil layer to separate out. After separation, it can be obtained by neutralization and distillation under reduced pressure. It can also be obtained by reacting terpineol, acetic anhydride and anhydrous sodium acetate, followed by neutralization, washing, separation and distillation.
2.Add the mixed aqueous solution of acetic acid and sulfuric acid into turpentine, place it at 30-40°C for several hours, and then allow the oil layer to separate out. After separation, it can be obtained by neutralization and distillation under reduced pressure. It can also be obtained by reacting terpineol, acetic anhydride and anhydrous sodium acetate, followed by neutralization, washing, separation and distillation.
3. Tobacco: FC, 18.
Purpose
1. Mainly used for preparing food flavor raw materials.
2.Mainly used in lavender, fragrant rose, cologne, pine needles, fruity fragrances and other daily fragrances; also used in grapefruit, citrus, It can be used in food flavors such as orange, peach, apricot, cherry, lemon, spicy and meaty flavors; it can also be used to add cardamom, sweet oregano, thyme and other flavoring essential oils to enhance Enhances its spicy aroma. The dosage used in chewing gum is 14~260mg/kg; in meat, it is 1.7~40mg /kg; condiments 15mg/kg; baked goods 15 mg/kg; 6.3mg/kg in candies; 3.5mg/ in soft drinks kg; 3.2mg/kg in cold drinks.

 微信扫一扫打赏
微信扫一扫打赏

