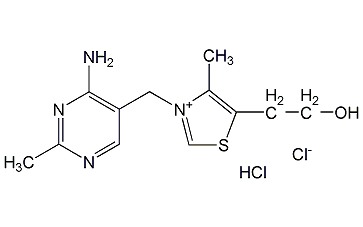
Structural formula
| Business number | 01EF |
|---|---|
| Molecular formula | C12H17ClN4OS·HCl |
| Molecular weight | 337.28 |
| label |
Thiamine hydrochloride, Aneurine hydrochloride, Vitamin B1 hydrochloride, Biochemical reagents |
Numbering system
CAS number:67-03-8
MDL number:MFCD00012780
EINECS number:200-641-8
RTECS number:XI7350000
BRN number:3851771
PubChem number:24900057
Physical property data
1.
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 250 (dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
p>
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC ): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. The upper limit of explosion (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Very soluble in water, 1 gram can be dissolved in 1 ml of water, 18 ml of propylene glycol, 100 ml of 95% ethanol, 315 ml of ethanol. Almost insoluble in ether, benzene, hexane and chloroform
Toxicological data
MouseLD50 (mg/kg ): 89.2 Intravenous injection; 8224 Oral.
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Number of hydrogen bond donors: 3
2. Number of hydrogen bond acceptors: 5
3. Number of rotatable chemical bonds: 4
4. Number of tautomers: 3
5. Topological molecular polar surface area (TPSA): 75.9
6. Number of heavy atoms: 20
7. Surface charge: 0
8, Complexity: 269
9, Number of isotope atoms: 0
10, Determine the number of atomic stereocenters: 0
11. The number of uncertain atomic stereocenters: 0
12. The number of determined chemical bond stereocenters: 0
13. The uncertain chemical bond stereocenters Quantity: 0
14, Number of covalent bond units: 3
Properties and stability
1%Aqueous solutionpH is 3.13. It is relatively stable in pH2-4 aqueous solution. pH5.5 and above are unstable to heating and will be damaged by ultraviolet rays. break down. It is stable in the air in a dry state, absorbs moisture quickly and decomposes slowly when exposed to the air.
Storage method
This product should be sealed and stored in a cool, dark place.
Synthesis method
1. Natural products exist in rice bran, germ, yeast, beans, etc., and can be extracted from rice bran or yeast after hydrolysis. There are many methods of synthesis. It can be prepared from 4-amino-2-methyl-5-acetylaminomethylpyrimidine through hydrolysis, addition condensation, cyclization hydrolysis, oxidation, substitution and other steps.
2.It can be formed from 4-amino-2-methyl-5-acetylaminomethylpyrimidine through hydrolysis, addition condensation, cyclization hydrolysis, It is prepared through oxidation, replacement and other steps. Natural products exist in rice bran, germ, yeast, beans, etc., and can be extracted from rice bran or yeast after hydrolysis.
3.Vitamin B1 naturally exists in rice bran, bran, peanuts, lean meat and other foods. Thiamine hydrochloride is usually obtained by condensation, hydrolysis, condensation, neutralization, oxidation and acidification of excess acetamidine hydrochloride and α-dimethoxy-β-methoxypropionitrile.
Purpose
1. This product plays an important role in glucose metabolism. The energy of nervous tissue is mainly supplied by glycogen metabolism. Once there is a lack of vitamin B1, the energy supply will be blocked, lactic acid and pyruvate will accumulate, resulting in polyneuritis, abnormal sensation, limb weakness, neuromuscular soreness and atrophy, and myocardial metabolism disorder. , symptoms of cardiobacterial insufficiency such as palpitations, chest tightness, cardiac hypertrophy, liver stasis, and peripheral edema appear. Mild vitamin B1 deficiency causes loss of appetite, weight loss, and weakness. It is used for vitamin B1 deficiency, high fever, hyperthyroidism, large amounts of glucose infusion, systemic infection, diabetes and polyneuritis, etc. According to the “Hygienic Standards for the Use of Food Nutritional Enhancers (1993)” promulgated by the Ministry of Health of my country, vitamin B1 can be used in cereals, their products, and beverages at a dosage of 3-5 mg/kg. It can also be used in infant and young child foods. The dosage is 4-8 mg/kg. Mouse oral toxicity LD507700-15000 mg/kg.
2. Used in medicine and biochemical research, fluorescence and phosphorescence analysis of phosphorus, and fluorescence determination of mercury.
3.Vitamin B1 plays an important role in maintaining normal nerve conduction, normal activities of the heart and digestive system. When it is deficient, people are susceptible to diseases such as beriberi or polyneuritis. Our country stipulates that it can be used in infant food, and the usage amount is 4 to 8 mg/kg; in cereals and their preparations, ;”>The dosage used in products is 3.0~5.0mg/kg; the dosage used in drinking liquids and milk drinks is 1~2mg/kg.

 微信扫一扫打赏
微信扫一扫打赏

