Background and overview[1][2]
Dehydronitrosonifedipine is an impurity of nifedipine. Nifedipine is a dihydropyridine calcium antagonist that can selectively inhibit the transmembrane transport of calcium ions into cardiomyocytes and smooth muscle cells, and inhibit the release of calcium ions from intracellular stores without changing plasma calcium ion concentration. It is currently recognized as One of the safe and effective first-line antihypertensive drugs. Nifedipine was discovered in 1969 and approved for use in the United States in 1981. It is available as a generic medicine and is present on the World Health Organization’s Model List of Essential Medicines, one of the most important medicines required for basic health systems. Nifedipine is a dihydropyridine calcium antagonist and one of the calcium antagonists. It can selectively inhibit the transmembrane transport of calcium ions into cardiomyocytes and smooth muscle cells, and inhibit the release of calcium ions from intracellular stores without Change plasma calcium ion concentration.
Preparation method[1]
General steps:
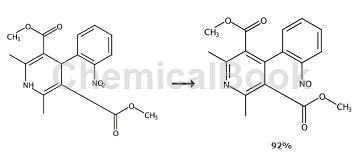
To a suspension of dihydropyridine (1.20 mmol) in acetic acid (4 mL) was added 0.1 mL of tris(bipyridyl)ruthenium(II) hexafluorophosphate (2 mg/mL, acetonitrile). This mixture was then irradiated with 3W blue LED light in a 15°C bath until TLC showed the reaction was complete. The solution was then diluted with water (20mL) and extracted with MTBE (20mL*3). Wash the combined organic phases with saturated aqueous sodium bicarbonate solution (10 mL *2) and brine (10 mL). The organic phase was concentrated to dryness and subjected to column chromatography to obtain the pyridine product.
Main reference materials
[1] Z. Lu et al. Photoinduced Aromatization of Dihydropyridines. Synthesis 2016, 48, 4221–4227
[2] CN201811305346.7 Nifedipine tablets and preparation method thereof

 微信扫一扫打赏
微信扫一扫打赏

