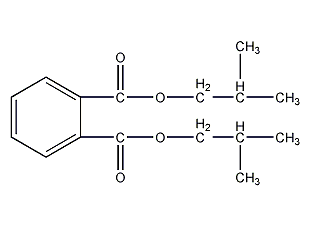
Structural formula
| Business number | 01UZ |
|---|---|
| Molecular formula | C16H22O4 |
| Molecular weight | 278.35 |
| label |
1,2-Di(2-methylpropyl)phthalate, Phthalic acid diisobutyl ester, 1,2-Benzenedicarboxylic acid,bis(2-methylpropyl)ester, Plasticizer |
Numbering system
CAS number:84-69-5
MDL number:MFCD00026480
EINECS number:201-553-2
RTECS number:TI1225000
BRN number:2054802
PubChem number:24887526
Physical property data
1. Properties: colorless transparent liquid
2. Density (g/cm3): 1.049
3. Boiling point (℃): 296.5
4. Flash point (℃): >110
5. Ignition point (℃): 182
6. Freezing point (℃): -50
7. Refractive index (n20D): 1.49
8. Viscosity (25℃, mPa·s): 36.4
9. Solubility: It has good compatibility with various cellulose and resins such as polyvinyl chloride, polystyrene, and polyvinyl acetate.
10. Melting point (ºC): -64
Toxicological data
1. Acute toxicity:
Rat caliber LD50: 15mg/kg;
Rat LD50: 20500mg/kg; Rat abdominal cavity LD50: 3750uL/kg;
p>
Mouse caliber LD50: 10mg/kg; mouse abdominal cavity LD50: 39900mg/kg;
Mouse LD50: 10500mg/kg;
Pig skin LD50: 10mg /kg;
2. Reproductive toxicity
Rat caliber TDL0: 8400 mg/kgSEX/DURATION; Rat abdominal TDL0: 1250 mg/kgSEX/DURATION
Mouse caliber TDL0: 16800 mg/kgSEX/DURATION; Mouse caliber TDL0: 32 mg/kgSEX/DURATION;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 77.51
2. Molar volume (cm3/mol): 265.0
3. Isotonic specific volume (90.2K): 650.4
4. Surface tension (dyne/cm): 36.2
5. Polarizability (10-24cm3): 30.72
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 290
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Existing in mainstream smoke.�
2. Pollute the environment.
Storage method
Store in a cool, ventilated warehouse away from light, away from fire sources, and protected from sun and rain. Prevent moisture intrusion.
Synthesis method
1. Phthalic anhydride and isobutanol undergo a normal pressure liquid phase esterification reaction under the catalysis of sulfuric acid. Monoisobutyl phthalate is first generated, and then the esterification is continued to generate diisobutyl phthalate. The mass ratio of phthalic anhydride and isobutanol is 1:1.35~1:1.4, and the sulfuric acid is 0.3% of the sum of the weight of phthalic anhydride and isobutanol. The esterification time takes 5 to 6 hours. The reactants are neutralized with 5% soda ash aqueous solution, vacuum distilled, excess isobutanol is recovered, and finally decolorized with activated carbon and filtered to obtain the finished product.
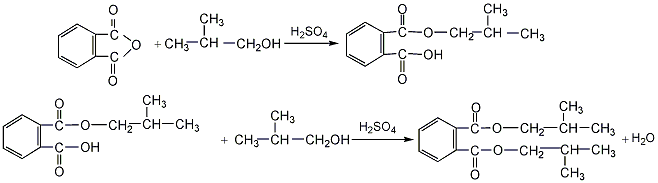
2.The process flow for preparing diisobutyl phthalate from phthalic anhydride residue is as follows: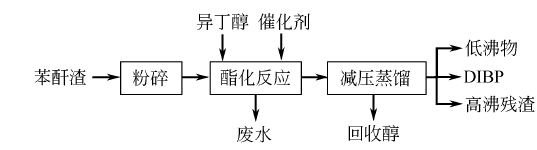
The process conditions are: the amount of catalyst is 10% of the total mass of the reactants, n (isobutanol) ∶n (phthalic anhydride) = 12:10, temperature 120~160℃, time 4h, the reaction is carried out under stirring. Distillation pressure ≤5.0kPa.
Purpose
1. It can be used as a plasticizer for cellulose resin, vinyl resin, nitrile rubber and chlorinated rubber. The plasticizing effect is similar to that of DBP, but its volatility and water extraction are greater than that of DBP, so it can be used as a substitute for DBP. This product is toxic to crops and is not suitable for use in agricultural PVC films. This product has low toxicity, and its oral LD50 in rats is 20-25g/kg. The United Kingdom, the Netherlands, and the United States allow this product to be used in food packaging materials. The United Kingdom stipulates that the maximum amount of this product in food packaging products made of polyvinyl chloride and vinyl chloride copolymer shall not exceed 40%.
2. It can be used as a plasticizer for polyvinyl chloride. The plasticizing effect is the same as that of di-n-butyl phthalate, but the volatility and water extraction loss are greater. It can be used as phthalic acid. Substitute for di-n-butyl ester. This product can also be used as a plasticizer for cellulose resin, vinyl resin, nitrile rubber and chloroprene rubber. This product is not suitable for use in the manufacture of agricultural films.
3. Used as plastic plasticizer. Has strong dissolving ability. It is mainly used in polyvinyl chloride plastics, but can also be used in rubber, adhesives and coatings. It has excellent softening effect and strong stability. It can also be used as a plasticizer for polyvinyl acetate, alkyd resin, ethyl cellulose and nitrocellulose. Its performance is similar to dibutyl phthalate, but its volatility and water extraction loss are large, and its durability is not ideal. This product has low toxicity and can be used as a plasticizer for food packaging materials.

 微信扫一扫打赏
微信扫一扫打赏

