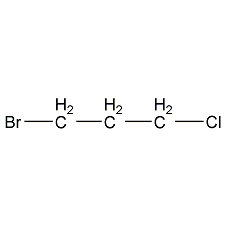
Structural formula
| Business number | 02ZK |
|---|---|
| Molecular formula | C3H6BrCl |
| Molecular weight | 157.44 |
| label |
1,3-bromochloropropane, 3-Chloro-1-bromopropane, 1-Chloro-3-bromopropane, 3-bromo-1-chloropropane, Trimethylene chloride bromide compound, 1,3-bromopropane, Trimethylene bromide chloride, Aliphatic halogenated derivatives |
Numbering system
CAS number:109-70-6
MDL number:MFCD00000998
EINECS number:203-697-1
RTECS number:TX4113000
BRN number:605278
PubChem number:24892149
Physical property data
1. Properties: colorless transparent liquid
2. Relative density (water=1): 1.5920
3. Relative vapor density (air=1): 5.5
p>
4. Melting point (ºC): -59
5. Boiling point (ºC, normal pressure): 144~145
6. Refractive index at room temperature (n20): 1.4866
7. Refractive index: 1.4864
8. Flash point (ºC): Undetermined
9. Specific rotation Degree (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (20ºC): 0.4719kpa
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined Determined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17 . Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: insoluble in water, Slightly soluble in glycerin, ether, ethanol, and chloroform.
Toxicological data
1. Acute toxicity: Rat oral LD50: 930mg/kg
Mouse oral LD50: 1290mg/kg
Rat inhalation LD50: 5668mg/m3
p>
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. It can cause pollution to the atmosphere and water bodies, and is extremely destructive to the atmospheric ozone layer.
Molecular structure data
1. Molar refractive index: 28.52
2. Molar volume (cm3/mol): 102.6
3. Isotonic specific volume (90.2K ): 242.0
4. Surface tension (dyne/cm)��30.9
5. Polarizability (10-24cm3): 11.30
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 16.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is forbidden to come into contact with strong oxidants, strong alkali and magnesium.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the addition of α-chloropropene and hydrogen bromide. Inhale α-chloropropene into the bromination tank, add a small amount of benzoyl peroxide, and then pass hydrogen bromide into the bottom of the tank to control the reaction temperature. At 18°C, the end point is when the relative density reaches above 1.5. Wash the product with water, alkali wash (2% sodium hydroxide), then wash with water, separate the water layer, dehydrate with anhydrous calcium chloride for 24 hours, fractionate under normal pressure, separate out low boiling matter below 130°C, and then distill under reduced pressure , collect the fraction above 70℃ (21.3kPa) to obtain the finished product. Hydrogen bromide used in production is produced by the following method: add hydrobromic acid to sodium bromide, raise the temperature to 60°C, add sulfuric acid dropwise, and keep the reaction at 80-90°C to release hydrogen bromide gas.
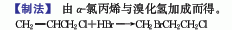
2. Preparation method:
p>
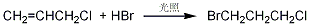
Add newly fractionated 3-Chloropropene (2) 38g (0.5mol), cooled in an ice-water bath, irradiated with a low-pressure ultraviolet lamp, slowly introduce hydrogen bromide gas equivalent to 1.1 to 1.2 times of the 3-chloropropene input amount, for about 2 to 8 hours over. The reaction solution was washed three times each with water and 3% sodium bicarbonate until neutral. Dry anhydrous calcium chloride overnight, and then fractionate under reduced pressure. First steam out the low boiling matter, and then collect the 68-69°C/7.9kPa fraction to obtain 61.5g of 1,3-bromochloropropane (1), with a yield of 78%. . [1]
Purpose
Used in the manufacture of trifluoperazine hydrochloride and organic synthesis. It is used as a pharmaceutical intermediate, mainly used in the synthesis of chlorpromazine, trifluoperazine, perphenazine, clomipramine, pyonazine, doxepin hydrochloride, Telden and other products.

 微信扫一扫打赏
微信扫一扫打赏

